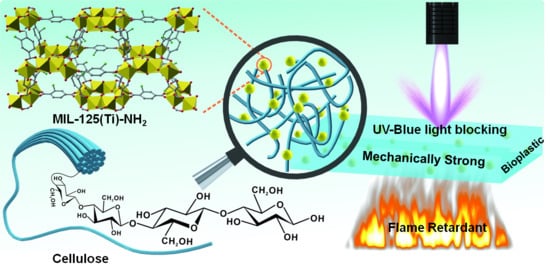
Nano-Metal Organic Framework for Enhanced Mechanical, Flame Retardant and Ultraviolet-Blue Light Shielding Properties of Transparent Cellulose-Based Bioplastics
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Reagents
2.2. Synthesis of Cellulose Hydrogel
2.3. Synthesis of MIL-125(Ti)-NH2@Cellulose Hydrogels (MNP@CHs)
2.4. Synthesis of MIL-125(Ti)-NH2@Cellulose Bioplastics (MNP@CBPs)
2.5. UV-Shielding Measurement of MNP@CBPs
2.6. Characterizations
3. Results and Discussion
3.1. Structure and Morphology of MNP@CBP
3.2. FTIR Analysis
3.3. XPS Analysis
3.4. XRD Analysis
3.5. TGA Analysis
3.6. Suggested Mechanism of Interaction
3.7. UV-Blue Light Resistance of MNP@CBPs
3.8. Flame Retardant Property
3.9. Mechanical Property
3.10. Contact Angle and Barrier Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Zhou, X.; Ding, W.; Zhang, Z.; Wang, Q. Do microplastics affect biological wastewater treatment performance? Implications from bacterial activity experiments. ACS Sustain. Chem. Eng. 2019, 7, 20097–20101. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, C.; Li, X. Enhanced electrolyte retention capability of separator for lithium-ion battery constructed by decorating ZIF-67 on bacterial cellulose nanofiber. Cellulose 2021, 28, 3097–3112. [Google Scholar] [CrossRef]
- Yang, W.S.; Gao, Y.; Zuo, C.; Deng, Y.L.; Dai, H.Q. Thermally-induced cellulose nanofibril films with near-complete ultraviolet-blocking and improved water resistance. Carbohydr. Polym. 2019, 223, 115050. [Google Scholar] [CrossRef]
- Lu, P.; Xiao, H.; Zhang, W.; Gong, G. Reactive coating of soybean oil-based polymer on nanofibrillated cellulose film for water vapor barrier packaging. Carbohydr. Polym. 2014, 111, 524–529. [Google Scholar] [CrossRef]
- Liu, P.; Chen, M.; Xiong, C.; Cao, X.; Wang, H. Flexible and highly sensitive graphene/carboxymethyl cellulose films for bending sensing. J. Mater. Sci. Mater. Electron. 2020, 31, 14118–14127. [Google Scholar] [CrossRef]
- Gan, W.T.; Wang, Y.X.; Xiao, S.L.; Gao, R.N.; Shang, Y.; Xie, Y.J.; Liu, J.Q.; Li, J. Magnetically driven 3d cellulose film for improved energy efficiency in solar evaporation. ACS Appl. Mater. Interfaces 2021, 13, 7756–7765. [Google Scholar] [CrossRef] [PubMed]
- Sadeghifar, H.; Venditti, R.A.; Jur, J.S.; Gorga, R.E.; Pawlak, J.J. Cellulose-lignin biodegradable and flexible UV protection film. ACS Sustain. Chem. Eng. 2017, 5, 625–631. [Google Scholar] [CrossRef]
- Xu, D.; Wang, S.; Wang, Y.; Liu, Y.; Zhu, P. Preparation and mechanism of flame-retardant cotton fabric with phosphoramidate siloxane polymer through multistep coating. Polymers 2020, 12, 1538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Rui, T.; Lu, C.; Xu, H.; Wei, Z. Mechanically robust, flame-retardant and anti-bacterial nanocomposite films comprised of cellulose nanofibrils and magnesium hydroxide nanoplatelets in a regenerated cellulose matrix. Cellulose 2014, 21, 1859–1872. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Xu, D.; Jie, C.; Zhang, L. Facile construction of cellulose/montmorillonite nanocomposite biobased plastics with flame retardant and gas barrier properties. Cellulose 2015, 22, 3799–3810. [Google Scholar] [CrossRef]
- Farooq, M.; Sipponen, M.H.; Seppälä, A.; Österberg, M. Eco-friendly flame-retardant cellulose nanofibril aerogels by incorporating sodium bicarbonate. ACS Appl. Mater. Interfaces 2018, 10, 27407–27415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alongi, J.; Tata, J.; Frache, A. Hydrotalcite and nanometric silica as finishing additives to enhance the thermal stability and flame retardancy of cotton. Cellulose 2011, 18, 179–190. [Google Scholar] [CrossRef]
- Song, K.L.; Ganguly, I.; Eastin, I.; Dichiara, A.B. Lignin-modified carbon nanotube/graphene hybrid coating as efficient flame retardant. Int. J. Mol. Sci. 2017, 18, 2368. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Song, L.; Wang, Z.G.; Wang, Y.Q.; Wan, L.; Yao, J.F. Highly transparent graphene oxide/cellulose composite film bearing ultraviolet shielding property. Int. J. Biol. Macromol. 2019, 145, 663–667. [Google Scholar] [CrossRef]
- Huang, D.J.; Zheng, Y.T.; Quan, Q.L. Enhanced mechanical properties and UV-shield of carboxymethyl cellulose films with polydopamine-modified natural fibre-like palygorskite. Appl. Clay Sci. 2019, 183, 105314. [Google Scholar] [CrossRef]
- Parka, S.J.; Yang, H.K.; Moon, B.K. Ultraviolet to blue blocking and wavelength convertible films using carbon dots for interrupting eye damage caused by general lighting. Nano Energy 2019, 60, 87–94. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Fang, G.; Huang, C.; Rojas, O.J.; Pan, H. Highly transparent, strong and flexible films with modified cellulose nanofiber bearing UV shielding property. Biomacromolecules 2018, 19, 4565–4575. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zhang, S.Y.; Huang, W.; Guo, Z.P.; Huang, J.J.; Yang, H.J.; Ye, D.Z.; Xu, W.L.; Gu, S.J. Multi-functional cotton textiles design using in situ generating zeolitic imidazolate framework-67 (ZIF-67) for effective UV resistance, antibacterial activity, and self-cleaning. Cellulose 2021, 28, 5923–5935. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [Green Version]
- Abdelhameed, R.M.; Ananias, D.; Silva, A.M.S.; Rocha, J. Building light-emitting metal-organic frameworks by post-synthetic modification. ChemistrySelect 2017, 2, 136–139. [Google Scholar] [CrossRef]
- Song, W.Q.; Zhu, M.; Zhu, Y.F.; Zhao, Y.Z.; Yang, M.X.; Miao, Z.C.; Ren, H.P.; Ma, Q.; Qian, L.W. Zeolitic imidazolate framework-67 functionalized cellulose hybrid aerogel: An environmentally friendly candidate for dye removal. Cellulose 2020, 27, 2161–2172. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. Performance and plasticization behavior of polymer–mof membranes for gas separation at elevated pressures. J. Membr. Sci. 2014, 470, 166–177. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Apostolopoulou-Kalkavoura, V.; da Costa, M.V.T.; Bergstrom, L.; Stromme, M.; Xu, C. Elastic aerogels of cellulose nanofbers@metal–organic frameworks for thermal insulation and fire retardancy. Nano Micro Lett. 2020, 12, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabipour, H.; Nie, S.B.; Wang, X.; Song, L.; Hu, Y. Highly flame retardant zeolitic imidazole framework-8@cellulose composite aerogels as absorption materials for organic pollutants. Cellulose 2020, 27, 2237–2251. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, Z.; Mao, X.; Che, X.L.; Li, H.H.; Wang, Y.Y. Multifunctional textiles/metal−organic frameworks composites for efficient eltraviolet radiation blocking and noise reduction. ACS Appl. Mater. Interfaces 2020, 12, 55316–55323. [Google Scholar] [CrossRef] [PubMed]
- Emam, H.E.; Abdelhameed, R.M. Anti-UV radiation textiles designed by embracing with nano-MIL(Ti, In)–metal organic framework. ACS Appl. Mater. Interfaces 2017, 9, 28034–28045. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, W.; Guo, Z.; Zhang, S.; Gu, S. Robust fluorine-free colorful superhydrophobic PDMS/NH2-MIL-125(Ti)@cotton fabrics for improved ultraviolet resistance and efficient oil-water separation. Cellulose 2019, 26, 9335–9348. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid dissolution of cellulose in LiOH/urea and NaOH/urea aqueous solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, J.; Zhang, L.; Xu, M.; Cheng, H.; Han, C.C.; Kuga, S.; Xiao, J.; Xiao, R. A bioplastic with high strength constructed from a cellulose hydrogel by changing the aggregated structure. J. Mater. Chem. A 2013, 1, 6678–6686. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-Shahat, M. Fabrication of ZIF-67@MIL-125-NH2 nanocomposite with enhanced visible light photoreduction activity. J. Environ. Chem. Eng. 2019, 7, 103194. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A new photoactive crystalline highly porous titanium(lV) dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef]
- Kaur, M.; Mehta, S.K.; Kansal, S.K. Amine-functionalized titanium metal-organic framework (NH2-MIL-125(Ti)): A novel fluorescent sensor for the highly selective sensing of copper ions. Mater. Chem. Phys. 2020, 254, 123539. [Google Scholar] [CrossRef]
- Chen, L.; Hou, X.; Song, N.; Shi, L.; Ding, P. Cellulose/graphene bioplastic for thermal management: Enhanced isotropic thermally conductive property by three-dimensional interconnected graphene aerogel. Compos. Part A Appl. Sci. Manuf. 2017, 107, 189–196. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, K.; Zhang, Y.; Cong, Y.; Wang, Q. Fabrication of visible-light-active MR/NH2-MIL-125(Ti) homojunction with boosted photocatalytic performance. Chem. Eng. J. 2021, 412, 128722. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.Z.; Wu, Y.; Zeng, G.M.; Chen, X.H.; Leng, L.J.; Wu, Z.B.; Jiang, L.B.; Li, H. Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr(VI) reduction. J. Hazard. Mater. 2015, 286, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Emam, H.E.; Abdelhameed, R.M. In-situ modification of natural fabrics by Cu-BTC MOF for effective release of insect repellent (N,N-diethyl-3-methylbenzamide). J. Porous Mater. 2017, 24, 1175–1185. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-Deib, H.; El-Dars, F.; Ahmed, H.B.; Emam, H.E. Applicable strategy for removing of liquid fuel nitrogenated contaminants using MIL-53-NH2@natural fabric composites. Ind. Eng. Chem. Res. 2018, 57, 15054–15065. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Emam, H.E.; Rocha, J.; Silva, A. Cu-BTC metal-organic framework natural fabric composites for fuel purification. Fuel Process. Technol. 2017, 159, 306–312. [Google Scholar] [CrossRef]
- Zhu, H.; Parvinian, S.; Preston, C.; Vaaland, O.; Ruan, Z.; Hu, L. Transparent nanopaper with tailored optical properties. Nanoscale 2013, 5, 3787–3792. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Fang, Z.; Preston, C.; Li, Y.; Hu, L. Transparent paper: Fabrications, properties, and device applications. Energy Environ. Sci. 2013, 7, 269–287. [Google Scholar] [CrossRef]
- Zhang, W.; Jing, Z.; Shan, Y.; Ge, X.; Mu, X.; Jiang, Y.; Li, H.; Wu, P. Paper reinforced with regenerated cellulose: a sustainable and fascinating material with good mechanical performance, barrier properties and shape retention in water. J. Mater. Chem. A. 2016, 4, 17483–17490. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Li, L.; An, X.; Qian, X. Nano-Metal Organic Framework for Enhanced Mechanical, Flame Retardant and Ultraviolet-Blue Light Shielding Properties of Transparent Cellulose-Based Bioplastics. Polymers 2021, 13, 2433. https://doi.org/10.3390/polym13152433
Sun L, Li L, An X, Qian X. Nano-Metal Organic Framework for Enhanced Mechanical, Flame Retardant and Ultraviolet-Blue Light Shielding Properties of Transparent Cellulose-Based Bioplastics. Polymers. 2021; 13(15):2433. https://doi.org/10.3390/polym13152433
Chicago/Turabian StyleSun, Lijian, Limei Li, Xianhui An, and Xueren Qian. 2021. "Nano-Metal Organic Framework for Enhanced Mechanical, Flame Retardant and Ultraviolet-Blue Light Shielding Properties of Transparent Cellulose-Based Bioplastics" Polymers 13, no. 15: 2433. https://doi.org/10.3390/polym13152433