Effect of Electrosynthesis Potential on Nucleation, Growth, Adhesion, and Electronic Properties of Polypyrrole Thin Films on Fluorine-Doped Tin Oxide (FTO)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
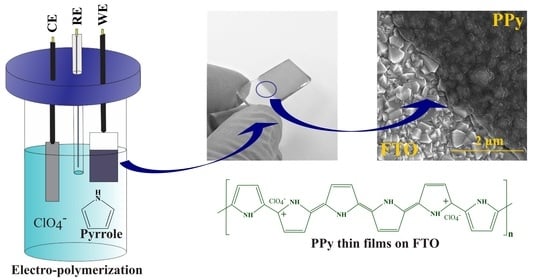
2.2. Electropolymerization Process
2.3. Characterizations
3. Results and Discussion
3.1. Electrosynthesis of Polypyrrole on FTO
3.2. Adhesion and Homogeneity of Polypyrrole Films on FTO
3.3. UV-Vis Absorption and Electronic Properties of Polypyrrole Films Electro-Synthesized on FTO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH) x. J. Chem. Soc. Chem. Commun. 1977, 36, 578. [Google Scholar] [CrossRef]
- Nguyen, V.A.; Kuss, C. Review—Conducting Polymer-Based Binders for Lithium-Ion Batteries and Beyond. J. Electrochem. Soc. 2020, 167, 065501. [Google Scholar] [CrossRef]
- Zang, X.; Wang, X.; Liu, H.; Ma, X.; Wang, W.; Ji, J.; Chen, J.; Li, R.; Xue, M. Enhanced Ion Conduction via Epitaxially Polymerized Two-Dimensional Conducting Polymer for High-Performance Cathode in Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 9347–9354. [Google Scholar] [CrossRef] [PubMed]
- Chhin, D.; Padilla-Sampson, L.; Malenfant, J.; Rigaut, V.; Nazemi, A.; Schougaard, S.B. Conducting Polymers Doped with Bifunctional Copolymers for Improved Organic Batteries. ACS Appl. Energy Mater. 2019, 2, 7781–7790. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, J.H.; Choi, S.-H. High-Performance Conducting Polymer/Si Nanowires Hybrid Solar Cells Using Multilayer-Graphene Transparent Conductive Electrode and Back Surface Passivation Layer. ACS Sustain. Chem. Eng. 2018, 6, 12446–12452. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hammed, W.A.; Yahya, R.B.; Mahmud, H.N.M.E. Prospects of conducting polymer and graphene as counter electrodes in dye-sensitized solar cells. J. Polym. Res. 2016, 23, 192. [Google Scholar] [CrossRef]
- Hu, X.; Meng, X.; Zhang, L.; Zhang, Y.; Cai, Z.; Huang, Z.; Su, M.; Wang, Y.; Li, M.; Li, F.; et al. A Mechanically Robust Conducting Polymer Network Electrode for Efficient Flexible Perovskite Solar Cells. Joule 2019, 3, 2205–2218. [Google Scholar] [CrossRef]
- Shen, K.-Y.; Hu, C.-W.; Chang, L.-C.; Ho, K.-C. A complementary electrochromic device based on carbon nanotubes/conducting polymers. Sol. Energy Mater. Sol. Cells 2012, 98, 294–299. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, B.; Li, X.; Xu, G.; Dou, S.; Zhang, X.; Chen, X.; Zhao, J.; Zhang, K.; Li, Y. Further understanding of the mechanisms of electrochromic devices with variable infrared emissivity based on polyaniline conducting polymers. J. Mater. Chem. C 2019, 7, 9878–9891. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Zhang, G.; Wang, S.; Zhu, S.; Wu, X.; Wang, Y.; Wang, Q.; Hu, C. Conducting polymer/silver nanowires stacking composite films for high-performance electrochromic devices. Sol. Energy Mater. Sol. Cells 2019, 200, 109919. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- El-Said, W.A.; Abdelshakour, M.; Choi, J.-H.; Choi, J.-W. Application of Conducting Polymer Nanostructures to Electrochemical Biosensors. Molecules 2020, 25, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratautaite, V.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. An Application of Conducting Polymer Polypyrrole for the Design of Electrochromic pH and CO 2 Sensors. J. Electrochem. Soc. 2019, 166, B297–B303. [Google Scholar] [CrossRef]
- Yuan, X.; Floresyona, D.; Aubert, P.-H.; Bui, T.-T.; Remita, S.; Ghosh, S.; Brisset, F.; Goubard, F.; Remita, H. Photocatalytic degradation of organic pollutant with polypyrrole nanostructures under UV and visible light. Appl. Catal. B Environ. 2019, 242, 284–292. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Z.-Y.; Zhao, H.; Yu, W.; Wu, S.; Liu, J.; Chen, L.; Li, Y.; Su, B.-L. Probing conducting polymers@cadmium sulfide core-shell nanorods for highly improved photocatalytic hydrogen production. J. Colloid Interface Sci. 2018, 521, 1–10. [Google Scholar] [CrossRef]
- Yuan, X.; Kobylanski, M.P.; Cui, Z.; Li, J.; Beaunier, P.; Dragoe, D.; Colbeau-Justin, C.; Zaleska-Medynska, A.; Remita, H. Highly active composite TiO2-polypyrrole nanostructures for water and air depollution under visible light irradiation. J. Environ. Chem. Eng. 2020, 8, 104178. [Google Scholar] [CrossRef]
- Yang, L.; Lv, M.; Song, Y.; Yin, K.; Wang, X.; Cheng, X.; Cao, K.; Li, S.; Wang, C.; Yao, Y.; et al. Porous Sn3O4 nanosheets on PPy hollow rod with photo-induced electrons oriented migration for enhanced visible-light hydrogen production. Appl. Catal. B Environ. 2020, 279, 119341. [Google Scholar] [CrossRef]
- Puerres, J.; Díaz, M.; Hurtado, J.; Ortiz, P.; Cortés, M.T. Photoelectrochemical Stability under Anodic and Cathodic Conditions of Meso-Tetra-(4-Sulfonatophenyl)-Porphyrinato Cobalt (II) Immobilized in Polypyrrole Thin Films. Polymers 2021, 13, 657. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, P. Conducting Polymers, Fundamentals and Applications; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-69376-7. [Google Scholar]
- Wan, M. Conducting Polymers with Micro or Nanometer Structure; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-69322-2. [Google Scholar]
- Shen, L.; Huang, X. Tuning the morphologies and electrical properties of azobenzene-4,4′-dicarboxylate-doped polypyrrole via ultraviolet light irradiation and medium pH alteration. Polymer 2019, 176, 188–195. [Google Scholar] [CrossRef]
- Morris, J.D.; Payne, C.K. Tuning PEDOT:PSS conductivity with iron oxidants. Org. Electron. 2014, 15, 1707–1710. [Google Scholar] [CrossRef]
- Waware, U.S.; Hamouda, A.M.S.; Hameed, A.S.; Summers, G.J. Tuning the electrical properties of polyaniline by copolymerization with o-bromoaniline. Funct. Mater. Lett. 2017, 10, 1750039. [Google Scholar] [CrossRef]
- Khodja, M.; El Kateb, M.; Beji, M.; Guittard, F.; Darmanin, T. Tuning nanotubular structures by templateless electropolymerization with thieno [3,4-b] thiophene-based monomers with different substituents and water content. J. Colloid Interface Sci. 2020, 564, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lehr, I.L.; Quinzani, O.V.; Saidman, S.B. Comparative study of polypyrrole films electrosynthesized in alkaline and acid solutions. Mater. Chem. Phys. 2009, 117, 250–256. [Google Scholar] [CrossRef]
- Longo, G.; Pompeo, G.; Serra Moreno, J.; Panero, S.; Girasole, M.; Ronci, F.; Cricenti, A. Morphological characterization of innovative electroconductive polymers in early stages of growth. Surf. Coat. Technol. 2012, 207, 286–292. [Google Scholar] [CrossRef]
- Janáky, C.; Rajeshwar, K. The role of (photo)electrochemistry in the rational design of hybrid conducting polymer/semiconductor assemblies: From fundamental concepts to practical applications. Prog. Polym. Sci. 2015, 43, 96–135. [Google Scholar] [CrossRef]
- Hwang, B.J.; Santhanam, R.; Lin, Y.-L. Nucleation and Growth Mechanism of Electropolymerization of Polypyrrole on Gold/Highly Oriented Pyrolytic Graphite Electrode. J. Electrochem. Soc. 2000, 147, 2252. [Google Scholar] [CrossRef]
- Hwang, B.J.; Santhanam, R.; Lin, Y.L. Nucleation and growth mechanism of electroformation of polypyrrole on a heat-treated gold/highly oriented pyrolytic graphite. Electrochim. Acta 2001, 46, 2843–2853. [Google Scholar] [CrossRef]
- Hwang, B.-J.; Santhanam, R.; Wu, C.-R.; Tsai, Y.-W. Nucleation and Growth Mechanism of Electropolymerization of Aniline on Highly Oriented Pyrolytic Graphite at a Low Potential. Electroanalysis 2001, 13, 37–44. [Google Scholar] [CrossRef]
- Hwang, B.-J.; Santhanam, R.; Lin, Y.-L. Evaluation of Structure, Nucleation and Growth Mechanism of Electropolymerized Polypyrrole on Highly Oriented Pyrolytic Graphite Electrode. Electroanalysis 2003, 15, 115–120. [Google Scholar] [CrossRef]
- Shustak, G.; Domb, A.J.; Mandler, D. n-Alkanoic Acid Monolayers on 316L Stainless Steel Promote the Adhesion of Electropolymerized Polypyrrole Films. Langmuir 2006, 22, 5237–5240. [Google Scholar] [CrossRef]
- Dian, G.; Merlet, N.; Barbey, G.; Outurquin, F.; Paulmier, C. Electrochemical polymerization of β-substituted and β,β’-disubstituted selenophenes. J. Electroanal. Chem. Interfacial Electrochem. 1987, 238, 225–237. [Google Scholar] [CrossRef]
- Mandić, Z.; Duić, L.; Kovačiček, F. The influence of counter-ions on nucleation and growth of electrochemically synthesized polyaniline film. Electrochim. Acta 1997, 42, 1389–1402. [Google Scholar] [CrossRef]
- Bruchlos, K.; Trefz, D.; Hamidi-Sakr, A.; Brinkmann, M.; Heinze, J.; Ruff, A.; Ludwigs, S. Poly(3-hexylthiophene) revisited—Influence of film deposition on the electrochemical behaviour and energy levels. Electrochim. Acta 2018, 269, 299–311. [Google Scholar] [CrossRef]
- Su, Y.-W.; Lin, W.-H.; Hsu, Y.-J.; Wei, K.-H. Conjugated Polymer/Nanocrystal Nanocomposites for Renewable Energy Applications in Photovoltaics and Photocatalysis. Small 2014, 10, 4427–4442. [Google Scholar] [CrossRef] [PubMed]
- Treat, N.D.; Chabinyc, M.L. Phase Separation in Bulk Heterojunctions of Semiconducting Polymers and Fullerenes for Photovoltaics. Annu. Rev. Phys. Chem. 2014, 65, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, W.; Liu, X.; Gu, Y. Research progress on polymer heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2012, 98, 129–145. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Castro-Beltran, A.; Dominguez, C.; Bahena-Uribe, D.; Sepulveda-Guzman, S.; Cruz-Silva, R. Effect of non-electroactive additives on the early stage pyrrole electropolymerization on indium tin oxide electrodes. Thin Solid Films 2014, 566, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Alsultan, M.; Choi, J.; Jalili, R.; Wagner, P.; Swiegers, G.F. Synergistic amplification of (photo)catalytic oxygen and hydrogen generation from water by thin-film polypyrrole composites. Mol. Catal. 2020, 490, 110955. [Google Scholar] [CrossRef]
- Yang, B.; Ma, D.; Zheng, E.; Wang, J. A self-rechargeable electrochromic battery based on electrodeposited polypyrrole film. Sol. Energy Mater. Sol. Cells 2019, 192, 1–7. [Google Scholar] [CrossRef]
- Andreoli, E.; Liao, K.-S.; Haldar, A.; Alley, N.J.; Curran, S.A. PPy:PSS as alternative to PEDOT:PSS in organic photovoltaics. Synth. Met. 2013, 185–186, 71–78. [Google Scholar] [CrossRef]
- Yang, B.; Yao, C.; Yu, Y.; Li, Z.; Wang, X. Nature Degradable, Flexible, and Transparent Conductive Substrates from Green and Earth-Abundant Materials. Sci. Rep. 2017, 7, 4936. [Google Scholar] [CrossRef] [Green Version]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging Transparent Electrodes Based on Thin Films of Carbon Nanotubes, Graphene, and Metallic Nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.I.; Blaine, T.; Gougam, A.B. Optical Transmission Enhancement of Fluorine Doped Tin Oxide (FTO) on Glass for Thin Film Photovoltaic Applications. Energy Procedia 2013, 42, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers—Persistent Models and New Concepts †. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Sabouraud, G.; Sadki, S.; Brodie, N. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Bof Bufon, C.C.; Vollmer, J.; Heinzel, T.; Espindola, P.; John, H.; Heinze, J. Relationship between Chain Length, Disorder, and Resistivity in Polypyrrole Films. J. Phys. Chem. B 2005, 109, 19191–19199. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Martinez, J.G. Electro-chemo-biomimetics from conducting polymers: Fundamentals, materials, properties and devices. J. Mater. Chem. B 2016, 4, 2069–2085. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.V.; Diaz, R.; Herrasti, P.; Ocon, P. Electrogeneration and Characterization of Poly(3-methylthiophene). Polym. J. 2001, 33, 514–521. [Google Scholar] [CrossRef]
- Raso, M.A.; González-Tejera, M.J.; Carrillo, I.; Sanchez de la Blanca, E.; García, M.V.; Redondo, M.I. Electrochemical nucleation and growth of poly-N-Methylpyrrole on copper. Thin Solid Films 2011, 519, 2387–2392. [Google Scholar] [CrossRef]
- González-Tejera, M.; Carrillo Ramiro, I.; Hernández-Fuentes, I. Nucleation and growth mechanism of polyfurane perchlorate doped films. Electrochim. Acta 2000, 45, 1973–1982. [Google Scholar] [CrossRef]
- Appel, G.; Schmeißer, D.; Bauer, J.; Bauer, M.; Egelhaaf, H.J.; Oelkrug, D. The formation of oligomers in the electrolyte upon polymerization of pyrrole. Synth. Met. 1999, 99, 69–77. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical deposition of conductive and adhesive polypyrrole-dopamine films. Sci. Rep. 2016, 6, 30475. [Google Scholar] [CrossRef] [Green Version]
- Brédas, J.L.; Scott, J.C.; Yakushi, K.; Street, G.B. Polarons and bipolarons in polypyrrole: Evolution of the band structure and optical spectrum upon doing. Phys. Rev. B 1984, 30, 1023–1025. [Google Scholar] [CrossRef]
- Rahaman, M.; Aldalbahi, A.; Almoiqli, M.; Alzahly, S. Chemical and Electrochemical Synthesis of Polypyrrole Using Carrageenan as a Dopant: Polypyrrole/Multi-Walled Carbon Nanotube Nanocomposites. Polymers 2018, 10, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foroughi, J.; Spinks, G.M.; Wallace, G.G. Effect of synthesis conditions on the properties of wet spun polypyrrole fibres. Synth. Met. 2009, 159, 1837–1843. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Jing, L.; Xie, M.; Song, Y.; Xu, H.; Li, H.; Xie, J. In situ construction efficient visible-light-driven three-dimensional Polypyrrole/Zn3In2S6 nanoflower to systematically explore the photoreduction of Cr(VI): Performance, factors and mechanism. J. Hazard. Mater. 2020, 384, 121480. [Google Scholar] [CrossRef] [PubMed]
- Midya, L.; Chettri, A.; Pal, S. Development of a Novel Nanocomposite Using Polypyrrole Grafted Chitosan-Decorated CDs with Improved Photocatalytic Activity under Solar Light Illumination. ACS Sustain. Chem. Eng. 2019, 7, 9416–9421. [Google Scholar] [CrossRef]
- Konwer, S.; Maiti, J.; Dolui, S.K. Preparation and optical/electrical/electrochemical properties of expanded graphite-filled polypyrrole nanocomposite. Mater. Chem. Phys. 2011, 128, 283–290. [Google Scholar] [CrossRef]
- Chaughtai, Z.; Hashmi, M.A.; Yar, M.; Ayub, K. Electronic structure of polypyrrole composited with a low percentage of graphene nanofiller. Phys. Chem. Chem. Phys. 2021. [Google Scholar] [CrossRef]
- Coelho, E.C.S.; Nascimento, V.B.; Ribeiro, A.S.; Navarro, M. Electrochemical and optical properties of new electrochromic and fluorescent nitrobenzoyl polypyrrole derivatives. Electrochim. Acta 2014, 123, 441–449. [Google Scholar] [CrossRef]
- Tokgöz, S.R.; Firat, Y.E.; Safi, Z.; Peksoz, A. Electrochemical Properties of Al Doped Polypyrrole Composite Polymer: Mott-Schottky Approximation and Density Functional Theory. J. Electrochem. Soc. 2019, 166, G54–G60. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef]
- Winder, C.; Sariciftci, N.S. Low bandgap polymers for photon harvesting in bulk heterojunction solar cells. J. Mater. Chem. 2004, 14, 1077. [Google Scholar] [CrossRef]
- Roncali, J. Molecular Engineering of the Band Gap of π-Conjugated Systems: Facing Technological Applications. Macromol. Rapid Commun. 2007, 28, 1761–1775. [Google Scholar] [CrossRef]
- Korjenic, A.; Raja, K.S. Electrochemical Stability of Fluorine Doped Tin Oxide (FTO) Coating at Different pH Conditions. J. Electrochem. Soc. 2019, 166, C169–C184. [Google Scholar] [CrossRef]
- Patois, T.; Lakard, B.; Monney, S.; Roizard, X.; Fievet, P. Characterization of the surface properties of polypyrrole films: Influence of electrodeposition parameters. Synth. Met. 2011, 161, 2498–2505. [Google Scholar] [CrossRef]
- Otero, T.F. Conducting Polymers, Electrochemistry, and Biomimicking Processes. In Modern Aspects of Electrochemistry; Kluwer Academic Publishers: Boston, MA, USA, 2005; pp. 307–434. [Google Scholar]
- Alizadeh, N.; Tavoli, F. Enhancing electrochromic contrast and redox stability of nanostructure polypyrrole film doped by heparin as polyanion in different solvents. J. Polym. Sci. Part. A Polym. Chem. 2014, 52, 3365–3371. [Google Scholar] [CrossRef]







| Parameter | 0.9 V | 1.0 V | 1.1 V | 1.2 V |
|---|---|---|---|---|
| Jm* (mA/cm2) | 3.107 | 5.451 | 7.257 | 9.220 |
| tm* (s) | 11.92 | 4.47 | 2.00 | 1.67 |
| t0 (s) | 0.74 | 0.15 | 0.02 | 0.01 |
| J0 (µA/cm2) | 11 | 23 | 528 | 832 |
| Potential (V) | 3D Inst. (R2) | 3D Prog. (R2) | 2D Inst. (R2) | 2D Prog. (R2) | 3D Inst. (S) | 3D Prog. (S) | 2D Inst. (S) | 2D Prog. (S) |
|---|---|---|---|---|---|---|---|---|
| 0.9 | 0.36 | 0.97 | 0.82 | 0.87 | 0.32 | 0.07 | 0.17 | 0.14 |
| 1.0 | 0.32 | 0.96 | 0.81 | 0.87 | 0.34 | 0.08 | 0.17 | 0.14 |
| 1.1 | 0.72 | 0.92 | 0.97 | 0.43 | 0.19 | 0.10 | 0.06 | 0.24 |
| 1.2 | 0.73 | 0.90 | 0.97 | 0.34 | 0.18 | 0.11 | 0.05 | 0.24 |
| Polymerization Potential (V) | Polymerization Charge (mC/m2) | HOMO (eV) | Relative Standard Deviation (%) |
|---|---|---|---|
| 0.9 | 42 | −4.26 | 0.9 |
| 1.0 | 42 | −4.36 | 0.9 |
| 1.1 | 42 | −4.42 | 0.9 |
| 1.2 | 42 | −4.53 | 0.9 |
| 0.9 | 21 | −4.14 | 2 |
| 1.0 | 21 | −4.26 | 1 |
| 1.1 | 21 | −4.32 | 1 |
| 1.2 | 21 | −4.43 | 2 |
| Polymerization Potential (V) | Sheet Resistance (Ω/sq) | Relative Standard Deviation (%) | Conductivity (S/m) | Relative Standard Deviation (%) |
|---|---|---|---|---|
| 0.9 | 90 | 2 | 1.27 × 104 | 2 |
| 1.0 | 143 | 1 | 8.0 × 103 | 1 |
| 1.1 | 2.9 × 102 | 3 | 4.0 × 103 | 2 |
| 1.2 | 6.6 × 102 | 3 | 1.7 × 103 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puerres, J.; Ortiz, P.; Cortés, M.T. Effect of Electrosynthesis Potential on Nucleation, Growth, Adhesion, and Electronic Properties of Polypyrrole Thin Films on Fluorine-Doped Tin Oxide (FTO). Polymers 2021, 13, 2419. https://doi.org/10.3390/polym13152419
Puerres J, Ortiz P, Cortés MT. Effect of Electrosynthesis Potential on Nucleation, Growth, Adhesion, and Electronic Properties of Polypyrrole Thin Films on Fluorine-Doped Tin Oxide (FTO). Polymers. 2021; 13(15):2419. https://doi.org/10.3390/polym13152419
Chicago/Turabian StylePuerres, Jhon, Pablo Ortiz, and María T. Cortés. 2021. "Effect of Electrosynthesis Potential on Nucleation, Growth, Adhesion, and Electronic Properties of Polypyrrole Thin Films on Fluorine-Doped Tin Oxide (FTO)" Polymers 13, no. 15: 2419. https://doi.org/10.3390/polym13152419