An Injectable Hybrid Gelatin Methacryloyl (GelMA)/Phenyl Isothiocyanate-Modified Gelatin (Gel-Phe) Bioadhesive for Oral/Dental Hemostasis Applications
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Synthesis of GelMA and Gel-Phe Polymers
2.3. Evaluation of the Water Solubility, Gel Formation, and Rheological and Mechanical Properties of GelMA/Gel-Phe Bioadhesives
2.4. In Vitro Burst Model
2.5. Identification of the Cytotoxicity of GelMA/Gel-Phe Bioadhesives
2.6. Blood Clotting Time Assay
2.7. In Vitro Porcine Skin and In Vitro Dental Bleeding Models
2.8. Statistical Analysis
3. Results and Discussion
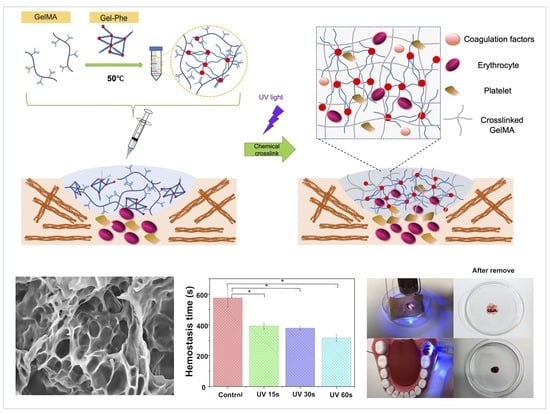
3.1. Design of Hybrid GelMA/Gel-Phe Bioadhesives
3.2. Synthesis and Characterization of GelMA/Gel-Phe
3.3. Evaluation of the Injectability and Mechanical Properties of Hybrid GelMA/Gel-Phe Bioadhesive
3.4. In Vitro Hemostastic Models for Examination of the GelMA/Gel-Phe Bioadhesives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, B.M.; Bortoto, J.B.; Fraga, G.P. Topical hemostatic agents in surgery: Review and prospects. Rev. Col. Bras. Cir. 2018, 45, e1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curnow, J.; Pasalic, L.; Favaloro, E.J. Why Do Patients Bleed? Surg. J. 2016, 2, e29–e43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaikh, M.S.; Zafar, M.S.; Pisani, F.; Lone, M.A.; Malik, Y.R. Critical features of periodontal flaps with regard to blood clot stability: A review. J. Oral. Biosci. 2021, 63, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, W.A.; Khalil, H. Dental extraction in patients on warfarin treatment. Clin. Cosmet. Investig. Dent. 2014, 6, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Liu, G.L.; Kaye, A.D.; Liu, H. Advances in Topical Hemostatic Agent Therapies: A Comprehensive Update. Adv. Ther. 2020, 37, 4132–4148. [Google Scholar] [CrossRef]
- Bhamidipati, C.M.; Coselli, J.S.; LeMaire, S.A. BioGlue in 2011: What is its role in cardiac surgery? J. Extra Corpor. Technol. 2012, 44, P6–P12. [Google Scholar]
- Wellisz, T.; Armstrong, J.K.; Cambridge, J.; An, Y.H.; Wen, X.; Kang, Q.; Hill, C.M.; Fisher, T.C. The effects of a soluble polymer and bone wax on sternal healing in an animal model. Ann. Thorac. Surg. 2008, 85, 1776–1780. [Google Scholar] [CrossRef]
- Ito, T.; Eriguchi, M.; Koyama, Y. Bioabsorbable bioadhesive hydrogel comprising poly(acrylic acid) and poly (vinylpyrrolidone) for adhesion barrier and hemostatic device. Mrs. Commun. 2015, 5, 291–295. [Google Scholar] [CrossRef]
- Hajosch, R.; Suckfuell, M.; Oesser, S.; Ahlers, M.; Flechsenhar, K.; Schlosshauer, B. A novel gelatin sponge for accelerated hemostasis. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 372–379. [Google Scholar] [CrossRef]
- Fan, X.; Li, M.; Yang, Q.; Wan, G.; Li, Y.; Li, N.; Tang, K. Morphology-controllable cellulose/chitosan sponge for deep wound hemostasis with surfactant and pore-foaming agent. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111408. [Google Scholar] [CrossRef]
- Hickman, D.A.; Pawlowski, C.L.; Sekhon, U.D.S.; Marks, J.; Gupta, A.S. Biomaterials and Advanced Technologies for Hemostatic Management of Bleeding. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhao, J.Y.; Hu, W.Z.; Ma, K.; Chao, Y.; Sun, P.J.; Fu, X.B.; Zhang, H. Synthetic poly(vinyl alcohol)-chitosan as a new type of highly efficient hemostatic sponge with blood-triggered swelling and high biocompatibility. J. Mater. Chem. B 2019, 7, 1855–1866. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, L.; Shi, X. Injectable drug-loaded polysaccharide hybrid hydrogels for hemostasis. RSC Adv. 2019, 9, 36858–36866. [Google Scholar] [CrossRef] [Green Version]
- Van den Broek, L.A.; Knoop, R.J.; Kappen, F.H.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef]
- Teuschl, A.H.; Zipperle, J.; Huber-Gries, C.; Kaplan, D.L. Silk fibroin based carrier system for delivery of fibrinogen and thrombin as coagulant supplements. J. Biomed. Mater. Res. A 2017, 105, 687–696. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.; Wang, X.; Zhang, M.; Li, C.; Zhou, J. Recent Advances on Synthetic and Polysaccharide Adhesives for Biological Hemostatic Applications. Front. Bioeng. Biotechnol. 2020, 8, 926. [Google Scholar] [CrossRef]
- Baik, S.H.; Kim, J.H.; Cho, H.H.; Park, S.N.; Kim, Y.S.; Suh, H. Development and analysis of a collagen-based hemostatic adhesive. J. Surg. Res. 2010, 164, e221–e228. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, S.M.; Bae, S.; Park, T.; Kim, H.; Jang, Y.; Moon, K.; Kim, H.; Lee, K.; Park, J.; et al. Effect of Aging on Homeostasis in the Soft Tissue of the Periodontium: A Narrative Review. J. Pers. Med. 2021, 11, 58. [Google Scholar] [CrossRef]
- Vyas, K.S.; Saha, S.P. Comparison of hemostatic agents used in vascular surgery. Expert Opin. Biol. Ther. 2013, 13, 1663–1672. [Google Scholar] [CrossRef] [Green Version]
- Mullins, R.J.; Richards, C.; Walker, T. Allergic reactions to oral, surgical and topical bovine collagen. Anaphylactic risk for surgeons. Aust. N. Z. J. Ophthalmol. 1996, 24, 257–260. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Akhlaq, M.; Azad, A.K.; Ullah, I.; Nawaz, A.; Safdar, M.; Bhattacharya, T.; Uddin, A.B.M.H.; Abbas, S.A.; Mathews, A.; Kundu, S.K.; et al. Methotrexate-Loaded Gelatin and Polyvinyl Alcohol (Gel/PVA) Hydrogel as a pH-Sensitive Matrix. Polymers 2021, 13, 2300. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.C.; Frost, M.C.; Zhao, F. Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, e2000905. [Google Scholar] [CrossRef]
- Noshadi, I.; Walker, B.W.; Portillo-Lara, R.; Shirzaei Sani, E.; Gomes, N.; Aziziyan, M.R.; Annabi, N. Engineering Biodegradable and Biocompatible Bio-ionic Liquid Conjugated Hydrogels with Tunable Conductivity and Mechanical Properties. Sci. Rep. 2017, 7, 4345. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Ostrovidov, S.; Ahadian, S.; Ramon-Azcon, J.; Hosseini, V.; Fujie, T.; Parthiban, S.P.; Shiku, H.; Matsue, T.; Kaji, H.; Ramalingam, M.; et al. Three-dimensional co-culture of C2C12/PC12 cells improves skeletal muscle tissue formation and function. J. Tissue Eng. Regen. Med. 2017, 11, 582–595. [Google Scholar] [CrossRef]
- Fares, M.M.; Shirzaei Sani, E.; Portillo Lara, R.; Oliveira, R.B.; Khademhosseini, A.; Annabi, N. Interpenetrating network gelatin methacryloyl (GelMA) and pectin-g-PCL hydrogels with tunable properties for tissue engineering. Biomater. Sci. 2018, 6, 2938–2950. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Wei, K.; Zhang, K.; Yang, B.; Tian, F.; Wang, G.; Bian, L. One-pot solvent exchange preparation of non-swellable, thermoplastic, stretchable and adhesive supramolecular hydrogels based on dual synergistic physical crosslinking. NPG Asia Mater. 2018, 10, e455. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Jodat, Y.A.; Samanipour, R.; Zorzi, G.; Zhu, K.; Hirano, M.; Chang, K.; Arnaout, A.; Hassan, S.; Matharu, N.; et al. Toward a neurospheroid niche model: Optimizing embedded 3D bioprinting for fabrication of neurospheroid brain-like co-culture constructs. Biofabrication 2020. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Zhang, Y.; Jiang, D.; Yang, L.; Lin, Q.; et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019, 10, 2060. [Google Scholar] [CrossRef]
- Assmann, A.; Vegh, A.; Ghasemi-Rad, M.; Bagherifard, S.; Cheng, G.; Sani, E.S.; Ruiz-Esparza, G.U.; Noshadi, I.; Lassaletta, A.D.; Gangadharan, S.; et al. A highly adhesive and naturally derived sealant. Biomaterials 2017, 140, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sun, X.; Zhang, K.; Yang, G.; Mu, Y.; Su, C.; Pang, J.; Chen, T.; Chen, X.; Feng, C. Chitosan/Diatom-Biosilica Aerogel with Controlled Porous Structure for Rapid Hemostasis. Adv. Healthc. Mater. 2020, 9, e2000951. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.C.; Chen, M.; Shi, Q.; Sun, R.; Wang, X. Chitosan/rectorite nanocomposite with injectable functionality for skin hemostasis. J. Mater. Chem. B 2018, 6, 6544–6549. [Google Scholar] [CrossRef]
- Sani, E.S.; Lara, R.P.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An Antimicrobial Dental Light Curable Bioadhesive Hydrogel for Treatment of Peri-Implant Diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef] [Green Version]
- Suneetha, M.; Rao, K.M.; Han, S.S. Mussel-Inspired Cell/Tissue-Adhesive, Hemostatic Hydrogels for Tissue Engineering Applications. ACS Omega 2019, 4, 12647–12656. [Google Scholar] [CrossRef] [Green Version]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Sharma, B.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Gupta, V.K.; Thakur, V.K. Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. J. Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springer, T.A.; Zhu, J.; Xiao, T. Structural basis for distinctive recognition of fibrinogen gammaC peptide by the platelet integrin alphaIIbbeta3. J. Cell. Biol. 2008, 182, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, A.; Bolte, M.; Erben, M.F.; Pérez, H. Intermolecular interactions in crystalline 1-(adamantane-1-carbonyl)-3-substituted thioureas with Hirshfeld surface analysis. CrystEngComm 2015, 17, 7551–7563. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, D.; David, J.F.; Chaudhuri, T.K.; Ray, A. Solution Processed Bismuth Ferrite Thin Films for All-Oxide Solar Photovoltaics. J. Phys. Chem. C 2015, 119, 5872–5877. [Google Scholar] [CrossRef]
- Valle, M.; Martin, L.; Maestro, A.; Andres, J.M.; Pedrosa, R. Chiral Bifunctional Thioureas and Squaramides Grafted into Old Polymers of Intrinsic Microporosity for Novel Applications. Polymers 2018, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [Green Version]
- Sabnis, A.; Rahimi, M.; Chapman, C.; Nguyen, K.T. Cytocompatibility studies of an in situ photopolymerized thermoresponsive hydrogel nanoparticle system using human aortic smooth muscle cells. J. Biomed. Mater. Res. A 2009, 91, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Wang, C.; Luo, W.; Li, P.; Li, S.; Yang, Z.; Hu, Z.; Liu, Y.; Ao, N. Preparation and evaluation of chitosan/alginate porous microspheres/Bletilla striata polysaccharide composite hemostatic sponges. Carbohydr. Polym. 2017, 174, 432–442. [Google Scholar] [CrossRef]
- Semberova, J.; De Paoli Lacerda, S.H.; Simakova, O.; Holada, K.; Gelderman, M.P.; Simak, J. Carbon nanotubes activate blood platelets by inducing extracellular Ca2+ influx sensitive to calcium entry inhibitors. Nano Lett. 2009, 9, 3312–3317. [Google Scholar] [CrossRef]
- Lacerda, S.H.; Semberova, J.; Holada, K.; Simakova, O.; Hudson, S.D.; Simak, J. Carbon nanotubes activate store-operated calcium entry in human blood platelets. ACS Nano 2011, 5, 5808–5813. [Google Scholar] [CrossRef]
- Gu, Z.; Yang, Z.; Wang, L.; Zhou, H.; Jimenez-Cruz, C.A.; Zhou, R. The role of basic residues in the adsorption of blood proteins onto the graphene surface. Sci. Rep. 2015, 5, 10873. [Google Scholar] [CrossRef] [Green Version]
- Thaler, U.; Deusch, E.; Kozek-Langenecker, S.A. In vitro effects of gelatin solutions on platelet function: A comparison with hydroxyethyl starch solutions. Anaesthesia 2005, 60, 554–559. [Google Scholar] [CrossRef]
- Rajabi, N.; Kharaziha, M.; Emadi, R.; Zarrabi, A.; Mokhtari, H.; Salehi, S. An adhesive and injectable nanocomposite hydrogel of thiolated gelatin/gelatin methacrylate/Laponite(R) as a potential surgical sealant. J. Colloid. Interface Sci. 2020, 564, 155–169. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Avery, R.K.; Assmann, A.; Paul, A.; McKinley, G.H.; Khademhosseini, A.; Olsen, B.D. Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage. ACS Nano 2014, 8, 9833–9842. [Google Scholar] [CrossRef] [Green Version]
- Ostomel, T.A.; Shi, Q.; Stucky, G.D. Oxide hemostatic activity. J. Am. Chem. Soc. 2006, 128, 8384–8385. [Google Scholar] [CrossRef]
- Wagner, W.R.; Pachence, J.M.; Ristich, J.; Johnson, P.C. Comparative in vitro analysis of topical hemostatic agents. J. Surg. Res. 1996, 66, 100–108. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Li, Z.X.; Wang, S.; Liu, C.S.; Meng, X.H.; Liu, C.G.; Lv, Y.H.; Yu, J. Preparation and blood coagulation evaluation of chitosan microspheres. J. Mater. Sci. Mater. Med. 2008, 19, 1371–1377. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Fibroblast encapsulation in gelatin methacryloyl (GelMA) versus collagen hydrogel as substrates for oral mucosa tissue engineering. J. Oral Biol. Craniofac. Res. 2020, 10, 573–577. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-C.; Tai, A.-Z.; Tsai, N.-Y.; Li, Y.-C.E. An Injectable Hybrid Gelatin Methacryloyl (GelMA)/Phenyl Isothiocyanate-Modified Gelatin (Gel-Phe) Bioadhesive for Oral/Dental Hemostasis Applications. Polymers 2021, 13, 2386. https://doi.org/10.3390/polym13142386
Chang W-C, Tai A-Z, Tsai N-Y, Li Y-CE. An Injectable Hybrid Gelatin Methacryloyl (GelMA)/Phenyl Isothiocyanate-Modified Gelatin (Gel-Phe) Bioadhesive for Oral/Dental Hemostasis Applications. Polymers. 2021; 13(14):2386. https://doi.org/10.3390/polym13142386
Chicago/Turabian StyleChang, Wan-Chun, Au-Zou Tai, Nian-Yun Tsai, and Yi-Chen Ethan Li. 2021. "An Injectable Hybrid Gelatin Methacryloyl (GelMA)/Phenyl Isothiocyanate-Modified Gelatin (Gel-Phe) Bioadhesive for Oral/Dental Hemostasis Applications" Polymers 13, no. 14: 2386. https://doi.org/10.3390/polym13142386