Tuning the Surface Wettability of Cyclic Olefin Copolymer by Plasma Treatment and Graphene Oxide Deposition and Reduction
Abstract
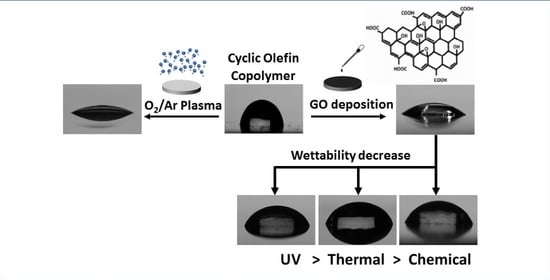
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO-Coated COC
2.3. Reduction of GO-Coated COC
2.3.1. UV Reduction
2.3.2. HI Reduction
2.3.3. Thermal Reduction
2.4. Characterization
3. Results
3.1. Effect of Plasma Exposure Time for COC
3.2. Structural Characterization of COC Coated with GO
3.3. Effect of Plasma Treatment on GO Coating
3.4. Effect of GO Concentration
3.5. Effect of Ultraviolet (UV), Hydroiodic Acid (HI) and Thermal Treatment on the Wettability of GO-Coated COC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hsu, S.-Y.; Zhang, Z.-Y.; Tsao, C.-W. Thermoplastic micromodel investigation of two-phase flows in a fractured porous medium. Micromachines 2017, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Bauer, W.-A.C.; Fischlechner, M.; Abell, C.; Huck, W.T. Hydrophilic PDMS microchannels for high-throughput formation of oil-in-water microdroplets and water-in-oil-in-water double emulsions. Lab Chip 2010, 10, 1814–1819. [Google Scholar] [CrossRef] [Green Version]
- Alazzam, A. Solution-based, flexible, and transparent patterned reduced graphene oxide electrodes for lab-on-chip applications. Nanotechnology 2019, 31, 075302. [Google Scholar] [CrossRef]
- Alazzam, A.; Alamoodi, N. Microfluidic Devices with Patterned Wettability Using Graphene Oxide for Continuous Liquid–Liquid Two-Phase Separation. ACS Appl. Nano Mater. 2020, 3, 3471–3477. [Google Scholar] [CrossRef]
- Carvalho, R.R.; Pujari, S.P.; Vrouwe, E.X.; Zuilhof, H. Mild and Selective C–H Activation of COC Microfluidic Channels Allowing Covalent Multifunctional Coatings. ACS Appl. Mater. Interfaces 2017, 9, 16644–16650. [Google Scholar] [CrossRef] [PubMed]
- Perez-Toralla, K.; Champ, J.; Mohamadi, M.R.; Braun, O.; Malaquin, L.; Viovy, J.-L.; Descroix, S. New non-covalent strategies for stable surface treatment of thermoplastic chips. Lab Chip 2013, 13, 4409–4418. [Google Scholar] [CrossRef]
- Tan, H.M.; Fukuda, H.; Akagi, T.; Ichiki, T. Surface modification of poly (dimethylsiloxane) for controlling biological cells’ adhesion using a scanning radical microjet. Thin Solid Film. 2007, 515, 5172–5178. [Google Scholar] [CrossRef]
- Silverio, V.; Canane, P.A.; Cardoso, S. Surface wettability and stability of chemically modified silicon, glass and polymeric surfaces via room temperature chemical vapor deposition. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 210–217. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, B.; Dai, Z.; Qin, J.; Lin, B. Grafting epoxy-modified hydrophilic polymers onto poly (dimethylsiloxane) microfluidic chip to resist nonspecific protein adsorption. Lab Chip 2006, 6, 942–947. [Google Scholar] [CrossRef]
- Roman, G.T.; Hlaus, T.; Bass, K.J.; Seelhammer, T.G.; Culbertson, C.T. Sol−gel modified poly (dimethylsiloxane) microfluidic devices with high electroosmotic mobilities and hydrophilic channel wall characteristics. Anal. Chem. 2005, 77, 1414–1422. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-Malek, C. Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment—An SEM investigation. Sens. Actuators B Chem. 2007, 123, 368–373. [Google Scholar] [CrossRef]
- Bausch, G.G.; Stasser, J.L.; Tonge, J.S.; Owen, M.J. Behavior of plasma-treated elastomeric polydimethylsiloxane coatings in aqueous environment. Plasmas Polym. 1998, 3, 23–34. [Google Scholar] [CrossRef]
- Lahann, J.; Balcells, M.; Lu, H.; Rodon, T.; Jensen, K.F.; Langer, R. Reactive polymer coatings: A first step toward surface engineering of microfluidic devices. Anal. Chem. 2003, 75, 2117–2122. [Google Scholar] [CrossRef]
- Hu, S.; Ren, X.; Bachman, M.; Sims, C.E.; Li, G.; Allbritton, N.L. Surface-directed, graft polymerization within microfluidic channels. Anal. Chem. 2004, 76, 1865–1870. [Google Scholar] [CrossRef]
- Abate, A.R.; Lee, D.; Do, T.; Holtze, C.; Weitz, D.A. Glass coating for PDMS microfluidic channels by sol–gel methods. Lab Chip 2008, 8, 516–518. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Bai, X.; Sun, X.; Wang, X.; Wang, E.; Dai, H. Highly conducting graphene sheets and Langmuir–Blodgett films. Nat. Nanotechnol. 2008, 3, 538–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, B.; Fu, L.; Liao, L.; Liu, N.; Yan, K.; Chen, Y.; Liu, Z. High-quality single-layer graphene via reparative reduction of graphene oxide. Nano Res. 2011, 4, 434–439. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312. [Google Scholar] [CrossRef] [Green Version]
- Alamoodi, N.; Alazzam, A. Droplet Coalescence by Selective Wettability Enhancement in Microfluidic Devices. Nanomaterials 2020, 10, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Lomeda, J.R.; Doyle, C.D.; Kosynkin, D.V.; Hwang, W.-F.; Tour, J.M. Diazonium functionalization of surfactant-wrapped chemically converted graphene sheets. J. Am. Chem. Soc. 2008, 130, 16201–16206. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Teoh, H.; Tao, Y.; Tok, E.; Ho, G.; Sow, C. Electrical current mediated interconversion between graphene oxide to reduced grapene oxide. Appl. Phys. Lett. 2011, 98, 173105. [Google Scholar] [CrossRef]
- Cote, L.J.; Cruz-Silva, R.; Huang, J. Flash reduction and patterning of graphite oxide and its polymer composite. J. Am. Chem. Soc. 2009, 131, 11027–11032. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Superhydrophobic composite films produced on various substrates. Langmuir 2008, 24, 11225–11232. [Google Scholar] [CrossRef]
- Wu, Z.; Han, H.; Han, W.; Kim, B.; Ahn, K.H.; Lee, K. Controlling the Hydrophobicity of Submicrometer Silica Spheres via Surface Modification for Nanocomposite Applications. Langmuir 2007, 23, 7799–7803. [Google Scholar] [CrossRef]
- Roy, S.; Yue, C.; Lam, Y.; Wang, Z.; Hu, H. Surface analysis, hydrophilic enhancement, ageing behavior and flow in plasma modified cyclic olefin copolymer (COC)-based microfluidic devices. Sens. Actuators B Chem. 2010, 150, 537–549. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Papa, I.; Coppola, G.; Lopresto, V.; Sansone, L.; Antonucci, V. Effect of Plasma Treatment on the Impact Behavior of Epoxy/Basalt Fiber-Reinforced Composites: A Preliminary Study. Polymers 2021, 13, 1293. [Google Scholar] [CrossRef]
- Zaharescu, T.; Jipa, S.; Gigante, B. Stabilized polyethylene on the sterilization dose range. Polym. Bull. 2006, 57, 729–735. [Google Scholar] [CrossRef]
- Yang, T.C.-K.; Lin, S.S.-Y.; Chuang, T.-H. Kinetic analysis of the thermal oxidation of metallocene cyclic olefin copolymer (mCOC)/TiO2 composites by FTIR microscopy and thermogravimetry (TG). Polym. Degrad. Stab. 2002, 78, 525–532. [Google Scholar] [CrossRef]
- Forsyth, J.; Perena, J.M.; Benavente, R.; Pérez, E.; Tritto, I.; Boggioni, L.; Brintzinger, H.H. Influence of the polymer microstructure on the thermal properties of cycloolefin copolymers with high norbornene contents. Macromol. Chem. Phys. 2001, 202, 614–620. [Google Scholar] [CrossRef]
- Abunahla, H.; Alamoodi, N.; Alazzam, A.; Mohammad, B. Micro-Pattern of Graphene Oxide Films Using Metal Bonding. Micromachines 2020, 11, 399. [Google Scholar] [CrossRef] [Green Version]
- Huh, S.H. Thermal reduction of graphene oxide. In Physics and Applications of Graphene—Experiments; Mikhailov, S., Ed.; InTech: Rijeka, Croatia, 2011; pp. 73–90. [Google Scholar]
- Chen, J.; Li, H.; Zhang, L.; Du, C.; Fang, T.; Hu, J. Direct reduction of graphene oxide/nanofibrillated cellulose composite film and its electrical conductivity research. Sci. Rep. 2020, 10, 3124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, C.K.; Pumera, M. Renewal of sp 2 bonds in graphene oxides via dehydrobromination. J. Mater. Chem. 2012, 22, 23227–23231. [Google Scholar] [CrossRef]















| Plasma Exposure Time (min) | Surface Roughness (nm) |
|---|---|
| 0 (untreated COC) | 15.7 |
| 5 | 33.4 |
| 20 | 43 |
| 120 | 122.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawaymeh, F.; Abbas, Y.; Khaleel, M.; Alazzam, A.; Alamoodi, N. Tuning the Surface Wettability of Cyclic Olefin Copolymer by Plasma Treatment and Graphene Oxide Deposition and Reduction. Polymers 2021, 13, 2305. https://doi.org/10.3390/polym13142305
Dawaymeh F, Abbas Y, Khaleel M, Alazzam A, Alamoodi N. Tuning the Surface Wettability of Cyclic Olefin Copolymer by Plasma Treatment and Graphene Oxide Deposition and Reduction. Polymers. 2021; 13(14):2305. https://doi.org/10.3390/polym13142305
Chicago/Turabian StyleDawaymeh, Fadi, Yawar Abbas, Maryam Khaleel, Anas Alazzam, and Nahla Alamoodi. 2021. "Tuning the Surface Wettability of Cyclic Olefin Copolymer by Plasma Treatment and Graphene Oxide Deposition and Reduction" Polymers 13, no. 14: 2305. https://doi.org/10.3390/polym13142305