Photodynamic Inactivation of Pseudomonas aeruginosa by PHEMA Films Loaded with Rose Bengal: Potentiation Effect of Potassium Iodide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
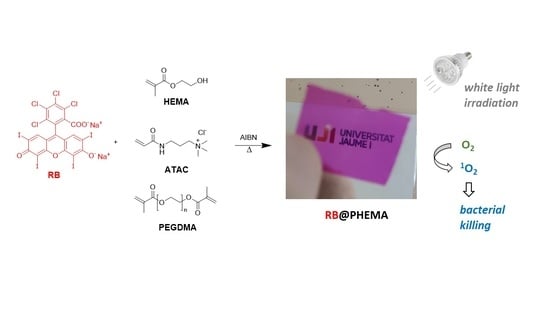
2.2. Hydrogel Synthesis
2.3. Characterization
2.4. Photochemical Studies
2.5. Microorganisms and Growth Conditions
2.6. Antimicrobial Photodynamic Inactivation Experiments
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of RB@PHEMA Films
3.2. Photochemical Generation of Singlet Oxygen by RB@PHEMA Films
3.3. Photodynamic Activity of RB@PHEMA Films against P. aeruginosa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, M.L. Changing patterns of infectious diseases. Nature 2000, 406, 762–767. [Google Scholar] [CrossRef]
- Nadimpalli, M.L.; Chan, C.W.; Doron, S. Antibiotic resistance: A call to action to prevent the next epidemic of inequality. Nat. Med. 2021, 27, 186–187. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Tambyah, P.A. Healthcare associated infections (HAI) perspectives. J. Infect. Public Health 2014, 7, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Huang, C.; Xiao, T.; Wu, Z.; Xiao, Y. A retrospective analysis of Pseudomonas aeruginosa bloodstream infections: Prevalence, risk factors, and outcome in carbapenem-susceptible and -non-susceptible infections. Antimicrob. Resist. Infect. Control. 2019, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Nigmatullin, R.; Gao, F. Onium-functionalised polymers in the design of non-leaching antimicrobial surfaces. Macromol. Mater. Eng. 2012, 297, 1038–1074. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur. Polym. J. 2015, 65, 46–62. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Ding, X.; Duan, S.; Ding, X.; Liu, R.; Xu, F.J. Versatile Antibacterial Materials: An Emerging Arsenal for Combatting Bacterial Pathogens. Adv. Funct. Mater. 2018, 28, 1–19. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8, 1–24. [Google Scholar] [CrossRef]
- Luo, H.; Yin, X.Q.; Tan, P.F.; Gu, Z.P.; Liu, Z.M.; Tan, L. Polymeric antibacterial materials: Design, platforms and applications. J. Mater. Chem. B 2021, 9, 2802–2815. [Google Scholar] [CrossRef] [PubMed]
- Koufakis, E.; Manouras, T.; Anastasiadis, S.H.; Vamvakaki, M. Film Properties and Antimicrobial Efficacy of Quaternized PDMAEMA Brushes: Short vs. Long Alkyl Chain Length. Langmuir 2020, 36, 3482–3493. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Recent progress in antimicrobial nanomaterials. Nanomaterials 2020, 10, 2315. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, M. Photodynamic Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. 2004, 3, 436–450. [Google Scholar] [CrossRef] [Green Version]
- Maisch, T. Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J. Photochem. Photobiol. B Biol. 2015, 150, 2–10. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R.; Abrahamse, H. Can light-based approaches overcome antimicrobial resistance? Drug Dev. Res. 2019, 80, 48–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakonieczna, J.; Wozniak, A.; Pieranski, M.; Rapacka-Zdonczyk, A.; Ogonowska, P.; Grinholc, M. Photoinactivation of ESKAPE pathogens: Overview of novel therapeutic strategy. Future Med. Chem. 2019, 11, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Song, Q.; Li, P.; Huang, W. Rejuvenated Photodynamic Therapy for Bacterial Infections. Adv. Healthc. Mater. 2019, 1900608, 1–19. [Google Scholar] [CrossRef]
- Decraene, V.; Pratten, J.; Wilson, M. Cellulose acetate containing toluidine blue and rose bengal is an effective antimicrobial coating when exposed to white light. Appl. Environ. Microbiol. 2006, 72, 4436–4439. [Google Scholar] [CrossRef] [Green Version]
- Noimark, S.; Dunnill, C.W.; Wilson, M.; Parkin, I.P. The role of surfaces in catheter-associated infections. Chem. Soc. Rev. 2009, 38, 3435–3448. [Google Scholar] [CrossRef]
- Spagnul, C.; Turner, L.C.; Boyle, R.W. Immobilized photosensitizers for antimicrobial applications. J. Photochem. Photobiol. B Biol. 2015, 150, 11–30. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting current photoactive materials for antimicrobial photodynamic therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henke, P.; Lang, K.; Kubát, P.; Sýkora, J.; Šlouf, M.; Mosinger, J. Polystyrene nanofiber materials modified with an externally bound porphyrin photosensitizer. ACS Appl. Mater. Interfaces 2013, 5, 3776–3783. [Google Scholar] [CrossRef]
- Martínez, S.R.; Palacios, Y.B.; Heredia, D.A.; Aiassa, V.; Bartolilla, A.; Durantini, A.M. Self-Sterilizing 3D-Printed Polylactic Acid Surfaces Coated with a BODIPY Photosensitizer. ACS Appl. Mater. Interfaces 2021, 13, 11597–11608. [Google Scholar] [CrossRef]
- Peddinti, B.S.T.; Scholle, F.; Ghiladi, R.A.; Spontak, R.J. Photodynamic Polymers as Comprehensive Anti-Infective Materials: Staying Ahead of a Growing Global Threat. ACS Appl. Mater. Interfaces 2018, 10, 25955–25959. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Pires, S.M.G.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Daniel-Da-Silva, A.L.; Almeida, A.; et al. Pyrrolidine-fused chlorin photosensitizer immobilized on solid supports for the photoinactivation of Gram negative bacteria. Dye. Pigment. 2014, 110, 123–133. [Google Scholar] [CrossRef]
- Wylie, M.P.; Irwin, N.J.; Howard, D.; Heydon, K.; McCoy, C.P. Hot-melt extrusion of photodynamic antimicrobial polymers for prevention of microbial contamination. J. Photochem. Photobiol. B Biol. 2021, 214, 112098. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.V.; Andrada, M.E.; Natera, J.; Muñoz, V.A.; Paulina Montãna, M.; Gambetta, C.; Boiero, M.L.; Montenegro, M.A.; Massad, W.A.; García, N.A. The employment of a removable chitosan-derivatized polymeric sensitizer in the photooxidation of polyhydroxylated water-pollutants. Photochem. Photobiol. 2014, 90, 1251–1256. [Google Scholar] [CrossRef] [Green Version]
- Aluigi, A.; Sotgiu, G.; Torreggiani, A.; Guerrini, A.; Orlandi, V.T.; Corticelli, F.; Varchi, G. Methylene Blue Doped Films of Wool Keratin with Antimicrobial Photodynamic Activity. ACS Appl. Mater. Interfaces 2015, 7, 17416–17424. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.L.; Scholle, F.; Sadeghifar, H.; Francis, A.J.; Boltersdorf, J.; Weare, W.W.; Argyropoulos, D.S.; Maggard, P.A.; Ghiladi, R.A. Synthesis, Characterization, and Antimicrobial Efficacy of Photomicrobicidal Cellulose Paper. Biomacromolecules 2015, 16, 2482–2492. [Google Scholar] [CrossRef]
- Stoll, K.R.; Scholle, F.; Zhu, J.; Zhang, X.; Ghiladi, R.A. BODIPY-embedded electrospun materials in antimicrobial photodynamic inactivation. Photochem. Photobiol. Sci. 2019, 18, 1923–1932. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Gomez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C. Imprinted contact lenses for ocular administration of antiviral drugs. Polymers 2020, 12, 2026. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Grochowicz, M.; Cauda, V. Porous ZnO/2-hydroxyethyl methacrylate eluting coatings for ureteral stent applications. Coatings 2018, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Rubakhin, S.S.; Enright, M.J.; Sweedler, J.V.; Nuzzo, R.G. 3D Particle-Free Printing of Biocompatible Conductive Hydrogel Platforms for Neuron Growth and Electrophysiological Recording. Adv. Funct. Mater. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Tian, Y.; Shumway, B.R.; Meldrum, D.R. A new cross-linkable oxygen sensor covalently bonded into poly(2-hydroxyethyl methacrylate)-co-polyacrylamide thin film for dissolved oxygen sensing. Chem. Mater. 2010, 22, 2069–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Wang, Q.; Chen, J.; Zhang, Q.; Zhao, X.; Qian, Y.; Zhu, C.; Yang, L.; Zhao, Y.; Kong, X.Y.; et al. Improved Ion Transport and High Energy Conversion through Hydrogel Membrane with 3D Interconnected Nanopores. Nano Lett. 2020, 20, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Subramani, R.; Huang, Y.T.; Lee, Y.L.; Jan, J.S.; Chiu, C.C.; Hou, S.S.; Teng, H. Highly stable interface formation in onsite coagulation dual-salt gel electrolyte for lithium-metal batteries. J. Mater. Chem. A 2021, 9, 5675–5684. [Google Scholar] [CrossRef]
- Horn, C.; Pospiech, D.; Allertz, P.J.; Müller, M.; Salchert, K.; Hommel, R. Chemical Design of Hydrogels with Immobilized Laccase for the Reduction of Persistent Trace Compounds in Wastewater. ACS Appl. Polym. Mater. 2021, 3, 2823–2834. [Google Scholar] [CrossRef]
- Galindo, F.; Lima, J.C.; Luis, S.V.; Parola, A.J.; Pina, F. Write-read-erase molecular-switching system trapped in a polymer hydrogel matrix. Adv. Funct. Mater. 2005, 15, 541–545. [Google Scholar] [CrossRef]
- Galindo, F.; Lima, J.C.; Luis, S.V.; Melo, M.J.; Jorge Parola, A.; Pina, F. Water/humidity and ammonia sensor, based on a polymer hydrogel matrix containing a fluorescent flavylium compound. J. Mater. Chem. 2005, 15, 2840–2847. [Google Scholar] [CrossRef]
- Bru, M.; Burguete, M.I.; Galindo, F.; Luis, S.V.; Marín, M.J.; Vigara, L. Cross-linked poly(2-hydroxyethylmethacrylate) films doped with 1,2-diaminoanthraquinone (DAQ) as efficient materials for the colorimetric sensing of nitric oxide and nitrite anion. Tetrahedron Lett. 2006, 47, 1787–1791. [Google Scholar] [CrossRef]
- Burguete, M.I.; Fabregat, V.; Galindo, F.; Izquierdo, M.A.; Luis, S.V. Improved polyHEMA-DAQ films for the optical analysis of nitrite. Eur. Polym. J. 2009, 45, 1516–1523. [Google Scholar] [CrossRef]
- Fabregat, V.; Izquierdo, M.A.; Burguete, M.I.; Galindo, F.; Luis, S.V. Quantum dot-polymethacrylate composites for the analysis of NO x by fluorescence spectroscopy. Inorg. Chim. Acta 2012, 381, 212–217. [Google Scholar] [CrossRef]
- Fabregat, V.; Izquierdo, M.Á.; Burguete, M.I.; Galindo, F.; Luis, S.V. Nitric oxide sensitive fluorescent polymeric hydrogels showing negligible interference by dehydroascorbic acid. Eur. Polym. J. 2014, 55, 108–113. [Google Scholar] [CrossRef]
- Fabregat, V.; Burguete, M.I.; Galindo, F.; Luis, S.V. Influence of polymer composition on the sensitivity towards nitrite and nitric oxide of colorimetric disposable test strips. Environ. Sci. Pollut. Res. 2017, 24, 3448–3455. [Google Scholar] [CrossRef] [Green Version]
- López-López, N.; Muñoz Resta, I.; De Llanos, R.; Miravet, J.F.; Mikhaylov, M.; Sokolov, M.N.; Ballesta, S.; García-Luque, I.; Galindo, F. Photodynamic Inactivation of Staphylococcus aureus Biofilms Using a Hexanuclear Molybdenum Complex Embedded in Transparent polyHEMA Hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 6995–7003. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.P.; Craig, R.A.; McGlinchey, S.M.; Carson, L.; Jones, D.S.; Gorman, S.P. Surface localisation of photosensitisers on intraocular lens biomaterials for prevention of infectious endophthalmitis and retinal protection. Biomaterials 2012, 33, 7952–7958. [Google Scholar] [CrossRef] [Green Version]
- Castro, K.A.D.F.; Moura, N.M.M.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Faustino, M.d.A.F.; Cunha, Â.; Almeida Paz, F.A.; Mendes, R.F.; Almeida, A.; Freire, C.S.R.; et al. Synthesis and characterization of photoactive porphyrin and poly(2-hydroxyethyl methacrylate) based materials with bactericidal properties. Appl. Mater. Today 2019, 16, 332–341. [Google Scholar] [CrossRef]
- Plíštil, L.; Henke, P.; Kubát, P.; Mosinger, J. Anion exchange nanofiber materials activated by daylight with a dual antibacterial effect. Photochem. Photobiol. Sci. 2014, 13, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblina, M.R. Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrob. Agents Chemother. 2015, 59, 5203–5212. [Google Scholar] [CrossRef] [Green Version]
- Agazzi, M.L.; Durantini, J.E.; Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. A novel tricationic fullerene C60 as broad-spectrum antimicrobial photosensitizer: Mechanisms of action and potentiation with potassium iodide. Photochem. Photobiol. Sci. 2021, 20, 327–341. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, X.; Szewczyk, G.; El-Hussein, A.; Huang, Y.-Y.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Antimicrobial Photodynamic Inactivation Mediated by Rose Bengal in In Vitro and In Vivo Studies. Antimicrob. Agents Chemother. 2017, 61, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin. ACS Infect. Dis. 2017, 3, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Reynoso, E.; Quiroga, E.D.; Agazzi, M.L.; Ballatore, M.B.; Bertolotti, S.G.; Durantini, E.N. Photodynamic inactivation of microorganisms sensitized by cationic BODIPY derivatives potentiated by potassium iodide. Photochem. Photobiol. Sci. 2017, 16, 1524–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, G.P.M.S.; Faustino, A.F.; Almeida, A. An insight into the potentiation effect of potassium iodide on APDT efficacy. Front. Microbiol. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R.; Abrahamse, H. Inorganic salts and antimicrobial photodynamic therapy: Mechanistic conundrums? Molecules 2018, 23, 3190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; El-Hussein, A.; Xuan, W.; Hamblin, M.R. Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation. J. Photochem. Photobiol. B Biol. 2018, 178, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kubát, P.; Henke, P.; Mosinger, J. The effect of iodide and temperature on enhancing antibacterial properties of nanoparticles with an encapsulated photosensitizer. Colloids Surf. B Biointerfaces 2019, 176, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.A.D.F.; Brancini, G.T.P.; Costa, L.D.; Biazzotto, J.C.; Faustino, M.A.F.; Tomé, A.C.; Neves, M.G.P.M.S.; Almeida, A.; Hamblin, M.R.; Da Silva, R.S.; et al. Efficient photodynamic inactivation of Candida albicans by porphyrin and potassium iodide co-encapsulation in micelles. Photochem. Photobiol. Sci. 2020, 19, 1063–1071. [Google Scholar] [CrossRef]
- Park, D.; Keszler, B.; Galiatsatos, V.; Kennedy, J.P. Amphiphilic networks. XI. Mechanical properties and morphology. J. Appl. Polym. Sci. 1997, 66, 901–910. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Zagora, J.; Plachá, D.; Echeverría, C.; Chiloeches, A.; Fernández-García, M. Chemical hydrogels bearing thiazolium groups with a broad spectrum of antimicrobial behavior. Polymers 2020, 12, 2853. [Google Scholar] [CrossRef]
- Litman, Y.; Rodríguez, H.B.; San Román, E. Tuning the concentration of dye loaded polymer films for maximum photosensitization efficiency: Phloxine B in poly(2-hydroxyethyl methacrylate). Photochem. Photobiol. Sci. 2016, 15, 80–85. [Google Scholar] [CrossRef]
- Ezquerra Riega, S.D.; Rodríguez, H.B.; Román, E.S. Rose bengal in poly(2-hydroxyethyl methacrylate) thin films: Selfquenching by photoactive energy traps. Methods Appl. Fluoresc. 2017, 5, 14010. [Google Scholar] [CrossRef]
- Mirenda, M.; Dicelio, L.E.; Román, E.S. Effect of molecular interactions on the photophysics of Rose Bengal in polyelectrolyte solutions and self-assembled thin films. J. Phys. Chem. B 2008, 112, 12201–12207. [Google Scholar] [CrossRef]
- Lacombe, S.; Pigot, T. Materials for selective photo-oxygenation vs. photocatalysis: Preparation, properties and applications in environmental and health fields. Catal. Sci. Technol. 2016, 6, 1571–1592. [Google Scholar] [CrossRef]
- Pereira, M.A.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Influence of external bacterial structures on the efficiency of photodynamic inactivation by a cationic porphyrin. Photochem. Photobiol. Sci. 2014, 13, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Merchat, M.; Bertolini, G.; Giacomini, P.; Villanueva, A.; Jori, G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B Biol. 1996, 32, 153–157. [Google Scholar] [CrossRef]
- Minnock, A.; Vernon, D.I.; Schofield, J.; Griffiths, J.; Parish, J.H.; Brown, S.B. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J. Photochem. Photobiol. B Biol. 1996, 32, 159–164. [Google Scholar] [CrossRef]
- Ragàs, X.; Sánchez-García, D.; Ruiz-González, R.; Dai, T.; Agut, M.; Hamblin, M.R.; Nonell, S. Cationic porphycenes as potential photosensitizers for antimicrobial photodynamic therapy. J. Med. Chem. 2010, 53, 7796–7803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soukos, N.S.; Ximenez-Fyvie, L.A.; Hamblin, M.R.; Socransky, S.S.; Hasan, T. Targeted Antimicrobial Photochemotherapy. Antimicrob. Agents Chemother. 1998, 42, 2595–2601. [Google Scholar] [CrossRef] [Green Version]
- Gavara, R.; de Llanos, R.; Pérez-Laguna, V.; Arnau del Valle, C.; Miravet, J.F.; Rezusta, A.; Galindo, F. Broad-Spectrum Photo-Antimicrobial Polymers Based on Cationic Polystyrene and Rose Bengal. Front. Med. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- del Valle, C.A.; Pérez-Laguna, V.; Resta, I.M.; Gavara, R.; Felip-León, C.; Miravet, J.F.; Rezusta, A.; Galindo, F. A cost-effective combination of Rose Bengal and off-the-shelf cationic polystyrene for the photodynamic inactivation of Pseudomonas aeruginosa. Mater. Sci. Eng. C 2020, 117, 111302. [Google Scholar] [CrossRef]
- Morrison, M.; Bayse, G.S.; Michaels, A.W. Determination of spectral properties of aqueous I2 and I3- and the equilibrium constant. Anal. Biochem. 1971, 42, 195–201. [Google Scholar] [CrossRef]









| Polymer | RB Loading (nmol RB/g Polymer) | T5% (°C) | T20% (°C) | Tmax (°C) | kobs103 (min−1) |
|---|---|---|---|---|---|
| A1 | 42 | 222.8 | 307.8 | 354.0 | 184 ± 39 a,b |
| A2 | 83 | 227.9 | 311.9 | 352.9 | 221 ± 41 b |
| A3 | 417 | 228.5 | 311.9 | 354.5 | 223 ± 48 b |
| A4 | 835 | 225.9 | 311.4 | 359.7 | 270 ± 30 b |
| B | 0 | 219.8 | 312.4 | 366.8 | 56 ± 13 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Fernández, A.M.; Muñoz Resta, I.; de Llanos, R.; Galindo, F. Photodynamic Inactivation of Pseudomonas aeruginosa by PHEMA Films Loaded with Rose Bengal: Potentiation Effect of Potassium Iodide. Polymers 2021, 13, 2227. https://doi.org/10.3390/polym13142227
López-Fernández AM, Muñoz Resta I, de Llanos R, Galindo F. Photodynamic Inactivation of Pseudomonas aeruginosa by PHEMA Films Loaded with Rose Bengal: Potentiation Effect of Potassium Iodide. Polymers. 2021; 13(14):2227. https://doi.org/10.3390/polym13142227
Chicago/Turabian StyleLópez-Fernández, Ana M., Ignacio Muñoz Resta, Rosa de Llanos, and Francisco Galindo. 2021. "Photodynamic Inactivation of Pseudomonas aeruginosa by PHEMA Films Loaded with Rose Bengal: Potentiation Effect of Potassium Iodide" Polymers 13, no. 14: 2227. https://doi.org/10.3390/polym13142227