Synthesis and Antimicrobial Properties of Highly Cross-Linked pH-Sensitive Hydrogels through Gamma Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
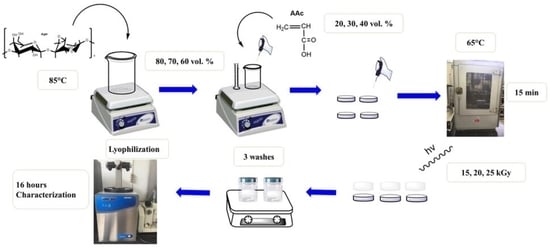
2.2. Preparation of Hydrogels
2.3. Synthesis of Silver Nanoparticles
2.4. Characterization
2.4.1. ATR-FTIR Analysis
2.4.2. Thermal Analysis
2.4.3. Mechanical Testing
2.4.4. Swelling Studies
2.4.5. Critical pH
2.4.6. Load of Antimicrobial Compounds
2.4.7. Antimicrobial Assay Using E. coli and MRSA
3. Results and Discussions
3.1. Synthesis of Hydrogels
3.2. Characterization
3.2.1. ATR-FTIR Analysis
3.2.2. Thermal Analysis
3.2.3. Mechanical Test
3.2.4. Swelling Studies
3.2.5. Critical pH
3.2.6. Load and Release of Ciprofloxacin
3.2.7. Load of Silver NPs
3.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hazra, C.; Kundu, D.; Chatterjee, A. Stimuli-responsive nanocomposites for drug delivery. In Applications of Nanocomposite Materials in Drug Delivery, 1st ed.; Inamuddin, A.A.M., Mohammad, A., Eds.; Elsevier Inc: Amsterdam, The Netherlands, 2018; pp. 823–841. ISBN 978-012-813-741-3. [Google Scholar]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Haltner-Ukomadu, E.; Sacha, M.; Richter, A.; Hussein, K. Hydrogel increases diclofenac skin permeation and absorption. Biopharm. Drug Dispos. 2019, 40, 2017–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirdirek, B.; Uhrich, K.E. Salicylic acid-based pH-sensitive hydrogels as potential oral insulin delivery systems. J. Drug Target. 2015, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Honeder, C.; Zhu, C.; Schöpper, H.; Gausterer, J.C.; Walter, M.; Landegger, L.D.; Saidov, N.; Riss, D.; Plasenzotti, R.; Gabor, F.; et al. Effects of sustained release dexamethasone hydrogels in hearing preservation cochlear implantation. Hear. Res. 2016, 341, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Dorati, R.; De Trizio, A.; Genta, I.; Merelli, A.; Modena, T.; Conti, B. Gentamicin-Loaded Thermosetting Hydrogel and Moldable Composite Scaffold: Formulation Study and Biologic Evaluation. J. Pharm. Sci. 2017, 106, 1596–1607. [Google Scholar] [CrossRef]
- Junker, J.P.E.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care. 2013, 2, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Vranckx, J.J.; Slama, J.; Preuss, S.; Perez, N.; Svensjö, T.; Visovatti, S.; Breuing, K.; Bartlett, R.; Pribaz, J.; Weiss, D.; et al. Wet wound healing. Plast. Reconstr. Surg. 2002, 110, 1680–1687. [Google Scholar] [CrossRef]
- Kirker, K.R.; Luo, Y.; Nielson, J.H.; Shelby, J.; Prestwich, G.D. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials 2002, 23, 3661–3671. [Google Scholar] [CrossRef]
- Hubbell, J.A. Hydrogel systems for barriers and local drug delivery in the control of wound healing. J. Control Release 1996, 39, 305–313. [Google Scholar] [CrossRef]
- Sullad, A.G.; Manjeshwar, L.S.; Aminabhavi, T.M. Novel pH-Sensitive Hydrogels Prepared from the Blends of Poly(vinyl alcohol) with Acrylic Acid-graft-Guar Gum Matrixes for Isoniazid Delivery. Ind. Eng. Chem. Res. 2010, 49, 7323–7329. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Zhang, S.; Xu, Z.; Lv, Z.-S.; Liu, M.-L.; Feng, L.-S. 4-Quinolone hybrids and their antibacterial activities. Eur. J. Med. Chem. 2017, 141, 335–345. [Google Scholar] [CrossRef]
- Weinsein, R.A.; Gaynes, R.; Edwards, J.R. Overview of Nosocomial Infections Caused by Gram-Negative Bacilli. Clin. Infect. Dis. 2005, 41, 848–854. [Google Scholar] [CrossRef]
- Ahn, S.; Kasi, R.M.; Kim, S.-C.; Sharma, N.; Zhou, Y. Stimuli-responsive polymer gels. Soft Matter 2008, 4, 1151–1157. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Dubruel, P.; Van Vlierberghe, S. Smart polymer hydrogels: Properties, synthesis, and applications. In Smart Polymers and their Applications, 1st ed.; Aguilar, M.R., Román, J.S., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2014; pp. 237–270. ISBN 978-085-709-702-6. [Google Scholar]
- You, J.-O.; Almeda, D.; Ye, G.J.C.; Auguste, D.T. Bioresponsive matrices in drug delivery. J. Biol. Eng. 2010, 4, 15:1–15:12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grainger, S.J.; El-Sayed, M.E.H. Stimuli-sensitive particles for drug delivery. In Biologically-Responsive Hybrid Biomaterials, 1st ed.; Jabbari, E., Khademhosseini, A., Eds.; World Scientific: Singapore, 2010; pp. 171–190. ISBN 978-981-446-556-4. [Google Scholar]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Schoener, C.A.; Hutson, H.N.; Peppas, N.A. pH-responsive hydrogels with dispersed hydrophobic nanoparticles for the delivery of hydrophobic therapeutic agents. Polym. Int. 2012, 61, 874–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, X.-Q.; Yang, X.-M.; Li, P.; Zhang, Z.-G.; Schönherr, H.; Zhang, D.; Feng, C.-L. Novel pH-responsive hydrogels for controlled cell adhesion and triggered surface detachment. Soft Matter 2012, 8, 9539–9544. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Behrens, M.A.; Pedersen, J.S.; Birkedal, H. Self-Healing Mussel-Inspired Multi-pH-Responsive Hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef]
- Armisén, R.; Galatas, F. Agar. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 87–104. ISBN 978-184-569-587-3. [Google Scholar]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.-L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Gidley, M.J.; Reid, J.S.G. Galactomannans and Other Cell Wall Storage Polysaccharides in Seeds. In Food Polysaccharides Their Applications, 2nd ed.; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 181–209. ISBN 978-082-475-922-3. [Google Scholar]
- Lee, P.C.; Meisel, D. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- El-Arnaouty, M.B.; Abdel Ghaffar, A.M.; Aboulfotouh, M.E.; Taher, N.H.; Taha, A.A. Radiation synthesis and characterization of Poly(butyl methacrylate/acrylamide) copolymeric hydrogels and heparin controlled drug release. Polym. Bull. 2015, 72, 2739–2756. [Google Scholar] [CrossRef]
- El-Arnaouty, M.B.; Eid, M.; Abdel Ghaffar, A.M. Radiation Synthesis of Stimuli Responsive Micro-Porous Hydrogels for Controlled Drug Release of Aspirin. Polym. Plast. Technol. Eng. 2015, 54, 1215–1222. [Google Scholar] [CrossRef]
- Lim, L.Y.; Khor, E.; Koo, O. γ-irradiation of chitosan. J. Biomed. Mater. Res. 1998, 43, 282–290. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S.; Ha, S.S.; Alunda, B.; Noh, D.Y.; Lee, Y.J.; Kim, S.; Seol, J.H. Crosslinking Effect on Thermal Conductivity of Electrospun Poly(acrylic acid) Nanofibers. Polymers 2019, 11, 858. [Google Scholar] [CrossRef] [Green Version]
- Everaerts, A.I.; Clemens, L.M. Pressure sensitive adhesives. In Adhesion Science and Engineering, 1st ed.; Dillar, D.A., Pocius, A.V., Chaudhury, M., Eds.; Elsevier Science, B.V.: Amsterdam, The Netherlands, 2002; pp. 465–534. ISBN 978-044-451-140-9. [Google Scholar]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Kheirandish, S.; Jabbari, E. Effect of surface polarity on wettability and friction coefficient of silicone rubber/poly(acrylic acid) hydrogel composite. Colloid Polym. Sci. 2006, 284, 1411–1417. [Google Scholar] [CrossRef]
- Blank, I.H. Measurement of pH of the Skin Surface. J. Investig. Dermatol. 1939, 2, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Zlotogorski, A. Distribution of skin surface pH on the forehead and cheek of adults. Arch. Dermatol. Res. 1987, 279, 398–401. [Google Scholar] [CrossRef]
- Islas, L.; Alvarez-Lorenzo, C.; Magariños, B.; Concheiro, A.; Del Castillo, L.F.; Burillo, G. Singly and binary grafted poly(vinyl chloride) urinary catheters that elute ciprofloxacin and prevent bacteria adhesion. Int. J. Pharm. 2015, 488, 20–28. [Google Scholar] [CrossRef]
- Wang, C.; Han, W.; Tang, X.; Zhang, H. Evaluation of Drug Release Profile from Patches Based on Styrene–Isoprene–Styrene Block Copolymer: The Effect of Block Structure and Plasticizer. AAPS PharmSciTech. 2012, 13, 556–567. [Google Scholar] [CrossRef] [Green Version]
- Moazeni, E.; Gilani, K.; Sotoudegan, F.; Pardakhty, A.; Najafabadi, A.R.; Ghalandari, R.; Fazeli, M.R.; Jamalifar, H. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J. Microencapsul. 2010, 27, 618–627. [Google Scholar] [CrossRef]
- Cornaglia, G.; Pompei, R.; Dainelli, B.; Satta, G. In Vitro Activity of Ciprofloxacin against Aerobic Bacteria Isolated in a Southern European Hospital. Antimicrob. Agents Chemother. 1987, 31, 1651–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Olivares, D.; Bucio, E. N-Vinylcaprolactam grafting onto cotton gauze by gamma radiation for loading and controlled release of antibacterial silver nanoparticles. MRS Advances 2020, 5, 3227–3237. [Google Scholar] [CrossRef]
- Campoli-Richards, D.M.; Monk, J.P.; Price, A.; Benfield, P.; Todd, P.A.; Ward, A. Ciprofloxacin. A Review of its Antibacterial Activity, Pharmacokinetic Properties and Therapeutic Use. Drugs 1988, 35, 373–447. [Google Scholar] [CrossRef] [PubMed]











| Agar/AAc (v/v%) | Radiation Dose (kGy) | Radiation Dose (kGy) | Radiation Dose (kGy) |
|---|---|---|---|
| 80/20 | 15 | 20 | 25 |
| 70/30 | 15 | 20 | 25 |
| 60/40 | 15 | 20 | 25 |
| Sample (Hydrogel) | Radiation (kGy) | Young’s Modulus (MPa) | Tensile Strength (MPa) | Displacement at Break (mm) |
|---|---|---|---|---|
| 20% AAc | 25 | 0.28 ± 0.35 | 0.78 ± 0.51 | 45 |
| 30% AAc | 25 | 0.37 ± 0.40 | 0.78 ± 0.44 | 47 |
| 40% AAc | 25 | 0.37 ± 0.36 | 0.48 ± 0.28 | 45.3 |
| 20% AAc | 20 | 0.73 ± 0.13 | 0.43 ± 0.21 | 77.5 |
| 30% AAc | 20 | 0.49 ± 1.13 | 0.95 ± 0.17 | 67 |
| 40% AAc | 20 | 1.64 ± 0.24 | 0.86 ± 0.13 | 58 |
| 20% AAc | 15 | 0.95 ± 0.23 | 2.15 ± 0.44 | 149 |
| 30% AAc | 15 | 0.99 ± 0.31 | 1.01 ± 0.42 | 122 |
| 40% AAc | 15 | 3.25 ± 1.29 | 2.13 ± 0.04 | 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustamante-Torres, M.; Pino-Ramos, V.H.; Romero-Fierro, D.; Hidalgo-Bonilla, S.P.; Magaña, H.; Bucio, E. Synthesis and Antimicrobial Properties of Highly Cross-Linked pH-Sensitive Hydrogels through Gamma Radiation. Polymers 2021, 13, 2223. https://doi.org/10.3390/polym13142223
Bustamante-Torres M, Pino-Ramos VH, Romero-Fierro D, Hidalgo-Bonilla SP, Magaña H, Bucio E. Synthesis and Antimicrobial Properties of Highly Cross-Linked pH-Sensitive Hydrogels through Gamma Radiation. Polymers. 2021; 13(14):2223. https://doi.org/10.3390/polym13142223
Chicago/Turabian StyleBustamante-Torres, Moises, Victor H. Pino-Ramos, David Romero-Fierro, Sandra P. Hidalgo-Bonilla, Héctor Magaña, and Emilio Bucio. 2021. "Synthesis and Antimicrobial Properties of Highly Cross-Linked pH-Sensitive Hydrogels through Gamma Radiation" Polymers 13, no. 14: 2223. https://doi.org/10.3390/polym13142223