The Oleofobization of Paper via Plasma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Paper Samples
2.2. Plasma Enhanced Chemical Vapor Deposition (PECVD)
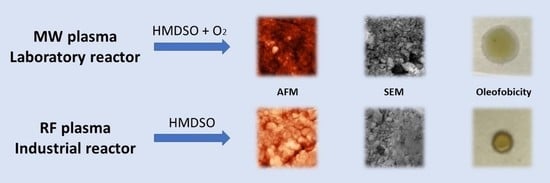
2.2.1. Laboratory Reactor
2.2.2. Industrial Reactor
2.3. Surface Morphology Analysis
2.3.1. Scanning Electron Microscopy
2.3.2. Atomic Force Microscopy
2.4. Surface Free Energy and Contact Angle
2.5. Surface Chemistry Analysis
2.5.1. X-ray Photoelectron Spectroscopy
2.5.2. Secondary Ion Mass Spectrometry
3. Results
3.1. Surface Morphology
3.2. Surface Free Energy and Hydrophilic/Oleophobic Properties
3.3. Surface Chemistry
3.3.1. XPS Analyses
3.3.2. SIMS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vartiainen, J.; Malm, T. Surface hydrophobization of CNF films by roll-to-roll HMDSO plasma deposition. J. Coat. Technol. Res. 2016, 13, 1145–1149. [Google Scholar] [CrossRef]
- Meunier, L.F.; Profili, J.; Babaei, S.; Asadollahi, S.; Sarkissian, A.; Dorris, A.; Beck, S.; Naudé, N.; Stafford, L. Modification of microfibrillated cellulosic foams in a dielectric barrier discharge at atmospheric pressure. Plasma Process. Polym. 2020, e2000158. [Google Scholar] [CrossRef]
- Babaei, S.; Profili, J.; Asadollahi, S.; Sarkassian, A.; Dorris, A.; Beck, S.; Stafford, L. Analysis of transport phenomena during plasma deposition of hydrophobic coatings on porous cellulosic substrates in plane-to-plane dielectric barrier discharges at atmospheric pressure. Plasma Process. Polym. 2020, 17, 2000091. [Google Scholar] [CrossRef]
- Rani, K.V.; Chandwani, N.; Kikani, P.; Nema, S.; Sarma, A.K.; Sarma, B. Hydrophobic surface modification of silk fabric using plasma-polymerized HMDSO. Surf. Rev. Lett. 2018, 25, 1850060. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic Coatings on Cellulose-Based Materials: Fabrication, Properties, and Applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]
- Gao, B.; Liu, H.; Gu, Z. Bottom-up fabrication of paper-based microchips by blade coating of cellulose microfibers on a patterned surface. Langmuir 2014, 30, 15041–15046. [Google Scholar] [CrossRef]
- Ražić, S.E.; Peran, J.; Kosalec, I. Functionalization of cellulose-based material by surface modifications using plasma and organosilicone/Ag compounds. In Proceedings of the ICNF 2015 from Nature to Market, 2nd International Conference on Natural Fibers, Azores, Portugal, 27–29 April 2015. [Google Scholar]
- Wang, Z.; Ma, H.; Chu, B.; Hsiao, B.S. Fabrication of cellulose nanofiber-based ultrafiltration membranes by spray coating approach. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Lyu, S.; Fu, F. Preparation of a robust cellulose nanocrystal superhydrophobic coating for self-cleaning and oil-water separation only by spraying. Ind. Crop. Prod. 2018, 122, 438–447. [Google Scholar] [CrossRef]
- Arbatan, T.; Zhang, L.; Fang, X.-Y.; Shen, W. Cellulose nanofibers as binder for fabrication of superhydrophobic paper. Chem. Eng. J. 2012, 210, 74–79. [Google Scholar] [CrossRef]
- Tomšič, B.; Simončič, B.; Orel, B.; Černe, L.; Tavčer, P.F.; Zorko, M.; Jerman, I.; Vilčnik, A.; Kovač, J. Sol-gel coating of cellulose fibres with antimicrobial and repellent properties. J. Sol-Gel Sci. Technol. 2008, 47, 44–57. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Liu, G.; Hu, H. Fluorine-free anti-smudge polyurethane coatings. Angew. Chem. 2015, 127, 12913–12918. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Aromaa, M.; Stepien, M.; Mäkelä, J.M.; Saarinen, J.J.; Toivakka, M.; Kuusipalo, J. High-and low-adhesive superhydrophobicity on the liquid flame spray-coated board and paper: Structural effects on surface wetting and transition between the low-and high-adhesive states. Colloid Polym. Sci. 2013, 291, 447–455. [Google Scholar] [CrossRef]
- Marchand, D.J.; Dilworth, Z.R.; Stauffer, R.J.; Hsiao, E.; Kim, J.-H.; Kang, J.-G.; Kim, S.H. Atmospheric rf plasma deposition of superhydrophobic coatings using tetramethylsilane precursor. Surf. Coat. Technol. 2013, 234, 14–20. [Google Scholar] [CrossRef]
- Pawlat, J.; Terebun, P.; Kwiatkowski, M.; Diatczyk, J. RF atmospheric plasma jet surface treatment of paper. J. Phys. D Appl. Phys. 2016, 49, 374001. [Google Scholar] [CrossRef]
- Martinu, L.; Poitras, D. Plasma deposition of optical films and coatings: A review. J. Vac. Sci. Technol. A Vac. Surf. Film. 2000, 18, 2619–2645. [Google Scholar] [CrossRef]
- Do Prado, M.; Da Silva, E.M.; das Neves Marques, J.; Gonzalez, C.B.; Simão, R.A. The effects of non-thermal plasma and conventional treatments on the bond strength of fiber posts to resin cement. Restor. Dent. Endod. 2017, 42, 125. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.J.; Church, J.S.; Huson, M.G. Pulsed plasma polymerization of hexamethyldisiloxane onto wool: Control of moisture vapor transmission rate and surface adhesion. Plasma Process. Polym. 2009, 6, 139–147. [Google Scholar] [CrossRef]
- Teske, M.; Wulf, K.; Fink, J.; Brietzke, A.; Arbeiter, D.; Eickner, T.; Senz, V.; Grabow, N.; Illner, S. Controlled biodegradation of metallic biomaterials by plasma polymer coatings using hexamethyldisiloxane and allylamine monomers. Curr. Dir. Biomed. Eng. 2019, 5, 315–317. [Google Scholar] [CrossRef] [Green Version]
- Lommatzsch, U.; Ihde, J. Plasma polymerization of HMDSO with an atmospheric pressure plasma jet for corrosion protection of aluminum and low-adhesion surfaces. Plasma Process. Polym. 2009, 6, 642–648. [Google Scholar] [CrossRef]
- Hsiao, C.-R.; Lin, C.-W.; Chou, C.-M.; Chung, C.-J.; He, J.-L. Surface modification of blood-contacting biomaterials by plasma-polymerized superhydrophobic films using hexamethyldisiloxane and tetrafluoromethane as precursors. Appl. Surf. Sci. 2015, 346, 50–56. [Google Scholar] [CrossRef]
- Gosar, Ž.; Kovač, J.; Mozetič, M.; Primc, G.; Vesel, A.; Zaplotnik, R. Deposition of SiOxCyHz protective coatings on polymer substrates in an industrial-scale PECVD reactor. Coatings 2019, 9, 234. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, A.; Barve, S.; Chutia, J.; Pal, A.; Kishore, R.; Pande, M.; Patil, D. RF-PACVD of water repellent and protective HMDSO coatings on bell metal surfaces: Correlation between discharge parameters and film properties. Appl. Surf. Sci. 2011, 257, 8469–8477. [Google Scholar] [CrossRef]
- Ji, Y.-Y.; Hong, Y.-C.; Lee, S.-H.; Kim, S.-D.; Kim, S.-S. Formation of super-hydrophobic and water-repellency surface with hexamethyldisiloxane (HMDSO) coating on polyethyleneteraphtalate fiber by atmosperic pressure plasma polymerization. Surf. Coat. Technol. 2008, 202, 5663–5667. [Google Scholar] [CrossRef]
- Odrásková, M.; Szalay, Z.; Ráhel, J.; Zahoranová, A.; Cernák, M. Diffuse coplanar surface barrier discharge assisted deposition of water repellent films from N2/HMDSO mixtures on wood surface. In Proceedings of the 28th International Conference on Phenomena in Ionized Gases, Prague, Czech Republic, 15–20 July 2007; pp. 15–20. [Google Scholar]
- Alexander, M.; Jones, F.; Short, R. Radio-frequency hexamethyldisiloxane plasma deposition: A comparison of plasma-and deposit-chemistry. Plasmas Polym. 1997, 2, 277–300. [Google Scholar] [CrossRef]
- Goujon, M.; Belmonte, T.; Henrion, G. OES and FTIR diagnostics of HMDSO/O2 gas mixtures for SiOx deposition assisted by RF plasma. Surf. Coat. Technol. 2004, 188, 756–761. [Google Scholar] [CrossRef]
- Hegemann, D.; Vohrer, U.; Oehr, C.; Riedel, R. Deposition of SiOx films from O2/HMDSO plasmas. Surf. Coat. Technol. 1999, 116, 1033–1036. [Google Scholar] [CrossRef]
- Hegemann, D.; Brunner, H.; Oehr, C. Deposition rate and three-dimensional uniformity of RF plasma deposited SiOx films. Surf. Coat. Technol. 2001, 142, 849–855. [Google Scholar] [CrossRef]
- Šourková, H.; Primc, G.; Špatenka, P.J.M. Surface functionalization of polyethylene granules by treatment with low-pressure air plasma. Materials 2018, 11, 885. [Google Scholar] [CrossRef] [Green Version]
- Gosar, Ž.; Kovač, J.; Mozetič, M.; Primc, G.; Vesel, A. Characterization of Gaseous Plasma Sustained in Mixtures of HMDSO and O2 in an Industrial-Scale Reactor. Plasma Chem. Plasma Process. 2020, 40, 25–42. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J.; King, R.C. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Nättinen, K.; Nikkola, J.; Minkkinen, H.; Heikkilä, P.; Lavonen, J.; Tuominen, M. Reel-to-reel inline atmospheric plasma deposition of hydrophobic coatings. J. Coat. Technol. Res. 2011, 8, 237–245. [Google Scholar] [CrossRef]
- Vartiainen, J.; Rose, K.; Kusano, Y.; Mannila, J.; Wikström, L. Hydrophobization, smoothing, and barrier improvements of cellulose nanofibril films by sol-gel coatings. J. Coat. Technol. Res. 2020, 17, 305–314. [Google Scholar] [CrossRef]














| Paper 1 | Paper 2 | |||||
|---|---|---|---|---|---|---|
| WCA (°) | SE (mN/m) | CA of Oil (°) | WCA (°) | SE (mN/m) | CA of Oil (°) | |
| Untreated | 71.4 (±6.7) | 48.8 (±3.9) | <5 | 115.0 (±4.0) | 31.2 (±1.3) | <5 |
| Laboratory plasma | 21.4 (±0.9) | 69.8 (±2.5) | <5 | 41.0 (±2.8) | 57.4 (±2.3) | <5 |
| Industrial plasma | 124.9 (±2.5) | 11.6 (±2.6) | 59.2 (±1.8) | 109.8 (±7.9) | 14.4 (±2.5) | 68.5 (±3.4) |
| Sample | C (at. %) | O (at. %) | Si (at. %) | Ca (at. %) |
|---|---|---|---|---|
| Untreated | 50.7 | 39.8 | 2.5 | 7.0 |
| Laboratory plasma | 5.1 | 65.4 | 29.6 | 0.0 |
| Industrial plasma | 44.5 | 30.1 | 25.4 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resnik, M.; Levičnik, E.; Gosar, Ž.; Zaplotnik, R.; Kovač, J.; Ekar, J.; Mozetič, M.; Junkar, I. The Oleofobization of Paper via Plasma Treatment. Polymers 2021, 13, 2148. https://doi.org/10.3390/polym13132148
Resnik M, Levičnik E, Gosar Ž, Zaplotnik R, Kovač J, Ekar J, Mozetič M, Junkar I. The Oleofobization of Paper via Plasma Treatment. Polymers. 2021; 13(13):2148. https://doi.org/10.3390/polym13132148
Chicago/Turabian StyleResnik, Matic, Eva Levičnik, Žiga Gosar, Rok Zaplotnik, Janez Kovač, Jernej Ekar, Miran Mozetič, and Ita Junkar. 2021. "The Oleofobization of Paper via Plasma Treatment" Polymers 13, no. 13: 2148. https://doi.org/10.3390/polym13132148