Halloysite Nanotubes and Silane-Treated Alumina Trihydrate Hybrid Flame Retardant System for High-Performance Cable Insulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Materials and Testing Samples
2.3. Characterization Methods of Polymer Compositions
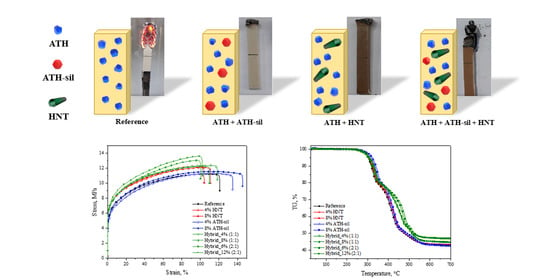
3. Results
3.1. Dispersion Characteristics
3.2. Mechanical and Processing Performance
3.3. Thermal Properties and Flame Retardancy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basfar, A.A. Flame retardancy of radiation cross-linked poly(vinyl chloride) (PVC) used as an insulating material for wire and cable. Polym. Degrad. Stabil. 2002, 77, 221–226. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Tang, G.; Shi, Y.; Hua, W.; Lu, H.; Song, L.; Hua, Y. Preparation of silane precursor microencapsulated intumescent flame retardant and its enhancement on the properties of ethylene-vinyl acetate copolymer cable. Compos. Sci. Technol. 2012, 72, 1042–1048. [Google Scholar] [CrossRef]
- Bahattab, M.A.; Mosnacek, J.; Basfar, A.A.; Shukri, T.M. Cross-linked poly(ethylene vinyl acetate) (EVA)/low density polyethylene (LDPE)/metal hydroxides composites for wire and cable applications. Polym. Bull. 2010, 64, 569–580. [Google Scholar] [CrossRef]
- Tan, Y.; Wachtendorf, V.; Kukofka, T.; Klack, P.; Ruder, J.; Lin, X.; Scharte, B. Degradation of flame retardance: A comparison of ethylene-vinyl acetate and low-density polyethylene cables with two different metal hydroxides. J. Appl. Polym. Sci. 2020, 138, 50149. [Google Scholar] [CrossRef]
- Liao, S.; Deng, C.; Huang, S.; Cao, J.; Wang, Y. An Efficient Halogen-free Flame Retardant for Polyethylene: Piperazine modified Ammonium Polyphosphates with Different Structures. Chin. J. Polym. Sci. 2016, 34, 1339–1353. [Google Scholar] [CrossRef]
- Qu, B.; Xie, R. Intumescent char structures and flame-retardant mechanism of expandable graphite-based halogen-free flame-retardant linear low density polyethylene blends. Polym. Int. 2003, 52, 1415–1422. [Google Scholar] [CrossRef]
- Ye, L.; Ding, P.; Zhang, M.; Qu, B. Synergistic Effects of Exfoliated LDH with Some Halogen-Free Flame Retardants in LDPE/EVA/HFMH/LDH Nanocomposites. J. Appl. Polym. Sci. 2008, 107, 3694–3701. [Google Scholar] [CrossRef]
- Luyt, A.S.; Malik, S.S.; Gasmi, S.A.; Porfyris, A.; Andronopoulou, A.; Korres, D.; Vouyiouka, S.; Grosshauser, M.; Pfaendner, R.; Brüll, R.; et al. Halogen-Free Flame-Retardant Compounds. Thermal Decomposition and Flammability Behavior for Alternative Polyethylene Grades. Polymers 2019, 11, 1479. [Google Scholar] [CrossRef] [Green Version]
- Arslan, F.; Dilsiz, N. Flame resistant properties of LDPE/PLA blends containing halogen-free flame retardant. J. Appl. Polym. Sci. 2020, 137, 48960. [Google Scholar] [CrossRef]
- Witkowski, A.; Stec, A.A.; Hull, T.R. The influence of metal hydroxide fire retardants and nanoclay on the thermal decomposition of EVA. Polym. Degrad. Stabil. 2012, 97, 2231–2240. [Google Scholar] [CrossRef]
- Yasir, M.; Amir, N.B.; Ahmad, F.; Rodzhan, N.S. Effect of basalt fibres reinforcement and aluminum trihydrate on the thermal properties of intumescent fire retardant coatings. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012185. [Google Scholar] [CrossRef] [Green Version]
- Beyer, G. Flame retardant properties of EVA-nanocomposites and improvements by combination of nanofillers with aluminium trihydrate. Fire Mater. 2001, 25, 193–197. [Google Scholar] [CrossRef]
- Beyer, G. Flame retardancy of nanocomposites based on organoclays and carbon nanotubes with aluminium trihydrate. Polym. Adv. Technol. 2006, 17, 218–225. [Google Scholar] [CrossRef]
- Konig, A.; Malek, A.; Fehrenbacher, U.; Brunklaus, G.; Wilhelm, M.; Hirth, T. Silane-functionalized Flame retardant Aluminum Trihydroxide in Flexible Polyurethane Foam. J. Cell. Plast. 2010, 46, 395–413. [Google Scholar] [CrossRef]
- Sadler, E.J.; Vecere, A.C. Silane treatment of mineral fillers—Practical aspects, Plastics. Rubber Compos. Process. Appl. 1995, 24, 271–275. [Google Scholar]
- Plueddemann, E.P. Silane Coupling Agents, Nature of Adhesion through Silane Coupling Agents; Springer Nature: Cham, Switzerland, 1991; pp. 115–152. [Google Scholar]
- Franciszczak, P.; Taraghi, I.; Paszkiewicz, S.; Burzynski, M.; Meljon, A.; Piesowicz, E. Effect of Halloysite Nanotube on Mechanical Properties, Thermal Stability and Morphology of Polypropylene and Polypropylene/Short Kenaf Fibers Hybrid Biocomposites. Materials 2020, 13, 4459. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Shi, X.; Liang, J.; Jia, Z.; Shi, G. Synergistic Effect of Halloysite Nanotubes on Flame Resistance of Intumescent Flame Retardant Poly(butylene succinate) Composites. Polym. Compos. 2019, 40, 202–209. [Google Scholar] [CrossRef]
- Saheb, S.M.; Tambe, P.; Malathi, M. Influence of halloysite nanotubes and intumescent flame retardant on mechanical and thermal properties of 80/20 (wt/wt) PP/ABS blend and their composites in the presence of dual compatibilizer. J. Thermo. Plast. Compos. Mater. 2018, 31, 202–222. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, C.L.; Du, S.L.; Chen, L.; Deng, C.; Wang, Y.Z. Synergistic Flame-Retardant Effect of Halloysite Nanotubes on Intumescent Flame Retardant in LDPE. J. Appl. Polym. Sci. 2014, 131, 40065. [Google Scholar] [CrossRef]
- Shen, L.; Li, J.; Li, R.; Lin, H.; Chen, J.; Liao, B.Q. A new strategy to produce low-density polyethylene (LDPE)-based composites simultaneously with high flame retardancy and high mechanical properties. Appl. Surf. Sci. 2018, 437, 75–81. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, X.; Kong, F.; Yang, J.; Nie, S. Investigation on novel flame retardant low-density polyethylene composites based on THEIC and MCAPP. J. Polym. Res. 2019, 26, 144. [Google Scholar] [CrossRef]
- Haurie, L.; Ferna´ndez, A.I.; Velasco, J.I.; Chimenos, J.M.; Cuesta, J.M.L.; Espiell, F. Thermal stability and flame retardancy of LDPE/EVA blends filled with synthetic hydromagnesite/aluminium hydroxide/montmorillonite and magnesium hydroxide/aluminium hydroxide/montmorillonite mixtures. Polym. Degrad. Stabil. 2007, 92, 1082–1087. [Google Scholar] [CrossRef]
- Feng, C.; Liang, M.; Jiang, J.; Huang, J.; Liu, H. Flame retardancy and thermal degradation behavior of efficient intumescent flame retardant LDPE composite containing 4A zeotile. J. Anal. Appl. Pyrol. 2016, 118, 9–19. [Google Scholar] [CrossRef]
- Chang, Z.H.; Guo, F.; Chen, J.F.; Yu, J.H.; Wang, G.Q. Synergistic flame retardant effects of nano-kaolin and nano-HAO on LDPE/EPDM composites. Polym. Degrad. Stabil. 2007, 92, 1204–1212. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, Y.; Song, P.; Cai, Y.; Guo, Q.; Fang, Z.; Peng, M. A novel zinc chelate complex containing both phosphorus and nitrogen for improving the flame retardancy of low density polyethylene. J. Anal. Appl. Pyrol. 2011, 92, 339–346. [Google Scholar] [CrossRef]
- Lu, C.; Gao, X.P.; Yao, D.H.; Cao, C.L.; Luo, Y.J. Improving flame retardancy of linear low-density polyethylene/nylon 6 blends via controlling localization of clay and intumescent flame-retardant. Polym. Degrad. Stabil. 2018, 153, 75–87. [Google Scholar] [CrossRef]
- Xu, J.; Ou, H.; Shan, X.; Liu, B.; Jiang, J.; Xu, G. Investigation of novel intumescent flame retardant low density polyethylene based on SiO2@MAPP and double pentaerythritol. J. Appl. Polym. Sci. 2020, 137, 49242. [Google Scholar] [CrossRef]
- Wu, Z.; Shu, W.; Hu, Y. Synergist Flame Retarding Effect of Ultrafine Zinc Borate on LDPE/IFR System. J. Appl. Polym. Sci. 2007, 103, 3667–3674. [Google Scholar] [CrossRef]
- Pandey, P.; Mohanty, S.; Nayak, S.K. Improved flame retardancy and thermal stability of polymer/clay nanocomposites, with the incorporation of multiwalled carbon nanotube as secondary filler: Evaluation of hybrid effect of nanofillers. High Perform. Polym. 2014, 26, 826–836. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Williams, G.R.; O’Hare, D.; Wang, Q. Layered double hydroxide-oxidized carbon nanotube hybrids as highly efficient flame retardant nanofillers for polypropylene. Sci. Rep. 2016, 6, 35502. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Wang, B.; Zhou, X.; Ma, J.; Yeb, Y.; Liu, C.; Xie, X. Multiple synergistic effects of graphene-based hybrid and hexagonal born nitride in enhancing thermal conductivity and flame retardancy of epoxy. Chem. Eng. J. 2020, 379, 122402. [Google Scholar] [CrossRef]
- Huang, S.C.; Deng, C.; Zhao, Z.Y.; Chen, H.C.; Gao, Y.Y.; Wang, Y.Z. Phosphorus-containing organic-inorganic hybrid nanoparticles for the smoke suppression and flame retardancy of thermoplastic polyurethane. Polym. Degrad. Stabil. 2020, 178, 109179. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Piesowicz, E.; Irska, I.; Kozlowski, L.; Wysocki, S.; Sieminski, J. The effect of the vinyl acetate content on the mechanical properties and flame retardancy of materials intended for the production of cable insulation. Przemysl. Chem. 2019, 98, 1153–1157. [Google Scholar]
- Gawdzinska, K.; Paszkiewicz, S.; Piesowicz, E.; Bryll, K.; Irska, I.; Lapis, A.; Sobolewska, E.; Kochmanska, A.; Slaczka, W. Preparation and Characterization of Hybrid Nanocomposites for Dental Applications. Appl. Sci. 2019, 9, 1381. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, C.Z.; Zhou, Q.; Shao, W. Aluminum hydroxide filled ethylene vinyl acetate (EVA) composites: Effect of the interfacial compatibilizer and the particle size. J. Mater. Sci. 2007, 42, 4227–4232. [Google Scholar] [CrossRef]
- Ismail, H.; Salleh, S.Z.; Ahmad, Z. Properties of halloysite nanotubes-filled natural rubber prepared using different mixing methods. Mater. Des. 2013, 50, 790–797. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Ismail, H.; Fauzi, M.N.A.; Bakar, A.A. Influence of maleic anhydride grafted ethylene propylene diene monomer (MAH-g-EPDM) on the properties of EPDM nanocomposites reinforced by halloysite nanotubes. Polym. Test. 2009, 28, 548–559. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Szymczyk, A.; Paszkiewicz, S.; Jedrzejewski, R.; Piesowicz, E.; Sieminski, J. Ethylene vinyl acetate copolymer/halloysite nanotubes nanocomposites with enhanced mechanical and thermal properties. J. Appl. Polym. Sci. 2020, 137, 49135. [Google Scholar] [CrossRef]
- Formela, K.; Korol, J.; Reza Saeb, M. Interfacially modified LDPE/GTR composites with non-polar elastomers: From microstructure to macro-behavior. Polym. Test. 2015, 42, 89–98. [Google Scholar] [CrossRef]
- Polansky, R.; Kadlec, P.; Slepicka, P.; Kolska, Z.; Svorcik, V. Testing the applicability of LDPE/HNT composites for cable core insulation. Polym. Test. 2019, 78, 105993. [Google Scholar]
- Szczygielska, A.; Kijenski, J. The Appl. ication of halloysite as a filler fr modification of polypropylene. Part I. The characterization of halloysite as a filler. Kompoz. Compos. 2010, 10, 181–185. [Google Scholar]
- Sikora, J.W.; Gajdoš, I.; Puszka, A. Polyethylene-Matrix Composites with Halloysite Nanotubes with Enhanced Physical/Thermal Properties. Polymers 2019, 11, 787. [Google Scholar] [CrossRef] [Green Version]
- Soares, V.L.P.; Nascimento, R.S.V.; Menezes, V.J.; Batista, L. TG characterization of organically modified montmorillonite. J. Therm. Anal. Calorim. 2004, 75, 671–676. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Silane Coupling Agents, 2nd ed.; Springer Science + Business Media: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Beyer, G. Flame Retardancy of Cables (Chapter 6) in the Global Cable Industry: Materials, Markets, Products; WILEY-VCH GmbH: Weinheim, Germany, 2020. [Google Scholar]
- Arao, Y. Flame Retardancy of Polymer Nanocomposite (Chapter 2) in Flame Retardants; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Tan, X.; He, Z.; Li, X.; Jiang, B.; Li, J.; Zhang, Y. Research on the Flame Retardancy Properties and Mechanism of Modified Asphalt with Halloysite Nanotubes and Conventional Flame Retardant. Materials 2020, 13, 4509. [Google Scholar] [CrossRef]









| Sample | EVA (%) | LLDPE (%) | Coupling Agent (%) | Plasticizer (%) | ATH Apyral(%) | Nanofillers | Thermal Stabilizer (%) | Stearin (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ELVAX 260 | ELVAX 40L | HNT (%) | ATH-sil (%) | |||||||
| Reference | 20.8 | 4 | 7.5 | 5 | 2 | 60 | - | - | 0.2 | 0.5 |
| 4%HNT | 20.8 | 4 | 7.5 | 5 | 2 | 56 | 4 | - | 0.2 | 0.5 |
| 8%HNT | 20.8 | 4 | 7.5 | 5 | 2 | 52 | 8 | - | 0.2 | 0.5 |
| 4%ATH-sil | 20.8 | 4 | 7.5 | 5 | 2 | 56 | - | 4 | 0.2 | 0.5 |
| 8%ATH-sil | 20.8 | 4 | 7.5 | 5 | 2 | 52 | - | 8 | 0.2 | 0.5 |
| Hybrid_4% (1:1) | 20.8 | 4 | 7.5 | 5 | 2 | 56 | 2 | 2 | 0.2 | 0.5 |
| Hybrid_8% (1:1) | 20.8 | 4 | 7.5 | 5 | 2 | 52 | 4 | 4 | 0.2 | 0.5 |
| Hybrid_6% (2:1) | 20.8 | 4 | 7.5 | 5 | 2 | 54 | 4 | 2 | 0.2 | 0.5 |
| Hybrid_12% (2:1) | 20.8 | 4 | 7.5 | 5 | 2 | 48 | 8 | 4 | 0.2 | 0.5 |
| Sample | TS (MPa) | Eb (%) | H (ShD) | Water Absorption (%) | MFI (21.6 kg/150 °C) |
|---|---|---|---|---|---|
| Reference | 11.22 ± 0.20 | 120.17 ± 5.71 | 38 ± 1 | 2.46 | 5.3 |
| 4%HNT | 12.06 ± 0.18 | 110.22 ± 7.35 | 41 ± 1 | 2.22 | 5.3 |
| 8%HNT | 12.23 ± 0.32 | 103.23 ± 5.54 | 41 ± 1 | 2.05 | 5.6 |
| 4%ATH-sil | 11.29 ± 0.17 | 134.12 ± 9.16 | 43 ± 1 | 2.17 | 5.5 |
| 8%ATH-sil | 11.55 ± 0.22 | 144.94 ± 11.38 | 44 ± 1 | 1.94 | 6.0 |
| Hybrid_4% (1:1) | 12.36 ± 0.13 | 117.80 ± 7.47 | 47 ± 1 | 1.92 | 5.9 |
| Hybrid_8% (1:1) | 12.25 ± 0.17 | 109.12 ± 7.24 | 51 ± 1 | 1.78 | 6.0 |
| Hybrid_6% (2:1) | 13.04 ± 0.23 | 110.24 ± 5.63 | 51 ± 1 | 1.26 | 6.1 |
| Hybrid_12% (2:1) | 13.38 ± 0.21 | 102.73 ± 4.85 | 54 ± 1 | 0.91 | 6.1 |
| Sample | VST (°C) | Tm(LDPE) (°C) | ΔHm (J/g) | Tc(LDPE) (°C) | ΔHc (J/g) | T5% (°C) | T50% (°C) |
|---|---|---|---|---|---|---|---|
| Reference | 60.5 ± 1.1 | 118.7 | 5.02 | 103.9 | 5.11 | 297 | 467 |
| 4%HNT | 59.8 ± 0.4 | 119.6 | 5.78 | 105.6 | 5.54 | 302 | 472 |
| 8%HNT | 60.4 ± 0.7 | 121.4 | 5.89 | 106.7 | 5.76 | 304 | 474 |
| 4%ATH-sil | 59.2 ± 1.3 | 120.2 | 5.81 | 105.2 | 5.80 | 311 | 473 |
| 8%ATH-sil | 60.2 ± 0.6 | 121.4 | 5.92 | 106.3 | 6.01 | 318 | 479 |
| Hybrid_4% (1:1) | 59.5 ± 0.6 | 120.5 | 5.84 | 105.9 | 5.79 | 302 | 506 |
| Hybrid_8% (1:1) | 61.5 ± 1.1 | 123.5 | 6.24 | 110.1 | 6.27 | 305 | 509 |
| Hybrid_6% (2:1) | 60.1 ± 1.0 | 122.7 | 6.12 | 109.3 | 6.03 | 297 | 513 |
| Hybrid_12% (2:1) | 61.5 ± 0.7 | 124.5 | 6.91 | 111.3 | 6.84 | 304 | 520 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszkiewicz, S.; Irska, I.; Taraghi, I.; Piesowicz, E.; Sieminski, J.; Zawisza, K.; Pypeć, K.; Dobrzynska, R.; Terelak-Tymczyna, A.; Stateczny, K.; et al. Halloysite Nanotubes and Silane-Treated Alumina Trihydrate Hybrid Flame Retardant System for High-Performance Cable Insulation. Polymers 2021, 13, 2134. https://doi.org/10.3390/polym13132134
Paszkiewicz S, Irska I, Taraghi I, Piesowicz E, Sieminski J, Zawisza K, Pypeć K, Dobrzynska R, Terelak-Tymczyna A, Stateczny K, et al. Halloysite Nanotubes and Silane-Treated Alumina Trihydrate Hybrid Flame Retardant System for High-Performance Cable Insulation. Polymers. 2021; 13(13):2134. https://doi.org/10.3390/polym13132134
Chicago/Turabian StylePaszkiewicz, Sandra, Izabela Irska, Iman Taraghi, Elżbieta Piesowicz, Jakub Sieminski, Karolina Zawisza, Krzysztof Pypeć, Renata Dobrzynska, Agnieszka Terelak-Tymczyna, Kamil Stateczny, and et al. 2021. "Halloysite Nanotubes and Silane-Treated Alumina Trihydrate Hybrid Flame Retardant System for High-Performance Cable Insulation" Polymers 13, no. 13: 2134. https://doi.org/10.3390/polym13132134