Differentiating Co-Delivery of Bisphosphonate and Simvastatin by Self-Healing Hyaluronan Hydrogel Formed by Orthogonal “Clicks”: An In-Vitro Assessment
Abstract
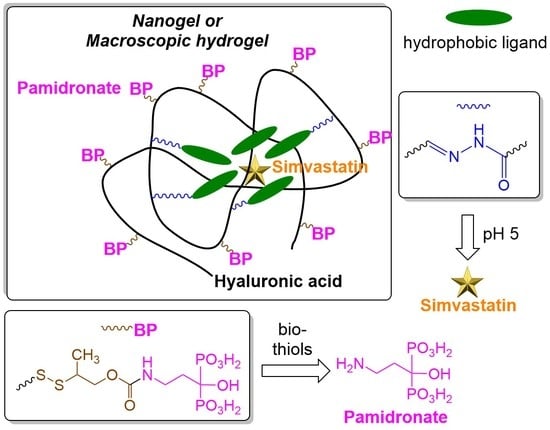
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 2-(2-Pyridyldithio)-2-Methylethanol 2
2.2. Synthesis of 2-(2-Pyridyldithio)-2-Methylethyl N-Hydroxysuccinimide Carbonate 3
2.3. Synthesis of Releasable 2-Pyridyldithio Derivative of Pamidronate 4
2.4. Synthesis of 2-Pyridyldithio Derivative of N-(6-Aminohexyl)-2,4-Dinitroaniline 5
2.5. Synthesis of Aldehyde-Modified DN Derivative 6
2.6. Synthesis of Releasable HA-//-BP Conjugate
2.7. Synthesis of Releasable BP-HA-//-DN Conjugate and Its Characterization by Dynamic Light Scattering (DLS)
2.8. Synthesis of hy-HA-//-BP Derivative
2.9. Synthesis of DN-hyd-HA-//-BP Derivative and Simvastatin Loading
2.10. Formation of HA-//-BP•Ca2+ Hydrogel and Its Thiol-Triggered Dissolution
2.11. Formation of DN-//-HA-BP•Ca2+ Hydrogel
2.12. Study of DN Release from DN-//-HA-BP•Ca2+ Hydrogel
2.13. Study of Simvastatin Release from SIM@DN-//-HA-BP•Ca2+ Nanogel
2.14. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Releasable HA-//-BP Conjugate
3.2. Coordination Hydrogel Formed by Releasable HA-//-BP
3.3. Synthesis and Characterization of Releasable BP-HA-//-DN Conjugate
3.4. Thiol-Triggered Release of Covalently Linked Drugs
3.5. Synthesis of DN-hyd-HA-//-BP Conjugate with Dual Release Mechanism
3.6. Acid-Triggered Release of DN Imaging Groups and Physically Encapsulated Simvastatin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coleman, R.E. Skeletal complications of malignancy. Cancer 1997, 80, 1588–1594. [Google Scholar] [CrossRef]
- Goltzman, D. Osteolysis and cancer. J. Clin. Investig. 2001, 107, 1219–1220. [Google Scholar] [CrossRef] [Green Version]
- Piperno-Neumann, S.; Le Deley, M.-C.; Redini, F.; Pacquement, H.; Marec-Bérard, P.; Petit, P.; Brisse, H.; Lervat, C.; Gentet, J.C.; Entz-Werlé, N.M.; et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1070–1080. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Rosen, C.J.; Bilezikian, J.P. Anabolic Therapy for Osteoporosis. J. Clin. Endocrinol. Metab. 2001, 86, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Leder, B.Z. Optimizing Sequential and Combined Anabolic and Antiresorptive Osteoporosis Therapy. J. Bone Miner. Res. 2018, 2, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abourehab, M.A.S. Hyaluronic Acid Modified Risedronate and Teriparatide Co-loaded Nanocarriers for Improved Osteogenic Differentiation of Osteoblasts for the Treatment of Osteoporosis. Curr. Pharm. Des. 2019, 25, 2975–2988. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Tao, L.; Abourehab, M.A.S.; Hussain, Z. Design and development of novel hyaluronate-modified nanoparticles for combo-delivery of curcumin and alendronate: Fabrication, characterization, and cellular and molecular evidences of enhanced bone regeneration. Int. J. Biol. Macromol. 2018, 116, 1268–1281. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Imitaz, N.; Niazi, M.B.K.; Fasim, F.; Khan, B.A.; Bano, S.A.; Shah, G.M.; Badshah, M.; Menaa, F.; Uzair, B. Fabrication of an Original Transparent PVA/Gelatin Hydrogel: In Vitro Antimicrobial Activity against Skin Pathogens. Int. J. Polym. Sci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Uzair, B.; Menaa, F.; Braga, V.A. Fabrication, Physical Characterization, and In Vitro, In Vivo Evaluation of Ginger Extract-Loaded Gelatin/Poly(Vinyl Alcohol) Hydrogel Films Against Burn Wound Healing in Animal Model. AAPS Pharm. Sci. Tech. 2020, 21, 323. [Google Scholar] [CrossRef]
- Russel, R.G.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.; Garrett, R.; Harris, S.; Chan, J.; Chen, D.; Rossini, G.; Boyce, B.; Zhao, M.; Gutierrez, G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999, 286, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.J.; Hart, D.J.; Spector, T.D. Oral statins and increased bone mineral density in postmenopausal women. Lancet 2000, 355, 2218–2219. [Google Scholar] [CrossRef]
- Dai, L.; Xu, M.; Wu, H.; Xue, L.; Yuan, D.; Wang, Y.; Shen, Z.; Zhao, H.; Hu, M. The functional mechanism of simvastatin in experimental osteoporosis. J. Bone Miner. Metab. 2016, 34, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Tikiz, C.; Tikiz, H.; Taneli, F.; Gümüşer, G.; Tüzün, Ç. Effects of simvastatin on bone mineral density and remodeling parameters in postmenopausal osteopenic subjects: 1-year follow-up study. Clin. Rheumatol. 2005, 24, 447–452. [Google Scholar] [CrossRef]
- Young, R.N.; Grynpas, M.D. Targeting therapeutics to bone by conjugation with bisphosphonates. Curr. Opin. Pharmacol. 2018, 40, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administration and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 256, 2649. [Google Scholar] [CrossRef]
- Menaa, F.; Menaa, A.; Menaa, B. Hyaluronic Acid and Derivatives for Tissue Engineering. J. Biotechnol. Biomater. 2011, S3. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, M.S.; Jeong, W.Y.; Han, D.-W.; Kim, K.S. Hyaluronic Acid-Based Theranostic Nanomedicines for Targeted Cancer Therapy. Cancers 2020, 12, 940. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Arns, S.; Young, R.N. Determination of the Rat in Vivo Pharmacokinetic profile of a Bone-Targeting Dual-Action Pro-Drug for Treatment of Osteoporosis. Bioconjugate Chem. 2015, 26, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, G.; Young, R.N. Design, Synthesis, and Pharmacokinetics of a Bone-Targeting Dual-Action prodrug for the Treatment of Osteoporosis. J. Med. Chem. 2017, 60, 7012–7028. [Google Scholar] [CrossRef]
- Pillow, T.H.; Sadowski, J.D.; Zhang, D.P.; Shang-Fan, Y.; Del Rosario, G.; Xu, K.; Xe, J.; Bhakta, S.; Ohri, R.; Kozak, K.R.; et al. Decoupling of stability and release in disulfide bonds with antibody-small molecule conjugates. Chem. Sci. 2017, 8, 366–370. [Google Scholar] [CrossRef] [Green Version]
- Morpurgo, M.; Bayer, E.A.; Wilchek, M. N-Hydroxysuccinimide carbonates and carbamates are useful reactive reagents for coupling ligands to lysines on proteins. J. Biochem. Biophys. Methods 1999, 38, 17–28. [Google Scholar] [CrossRef]
- Ossipov, D.A.; Kootala, S.; Yi, Z.; Yang, X.; Hilborn, J. Orthogonal Chemoselective Assembly of Hyaluronic Acid Networks and Nanogels for Drug Delivery. Macromolecules 2013, 46, 4105–4113. [Google Scholar] [CrossRef]
- Ossipov, D.A.; Yang, X.; Varghese, O.P.; Kootala, S.; Hilborn, J. Modular approach to functional hyaluronic acid hydrogels using orthogonal chemical reactions. Chem. Commun. 2010, 46, 8368–8370. [Google Scholar] [CrossRef]
- Kootala, S.; Zhang, Y.; Ghalib, S.; Tolmachev, V.; Hilborn, J.; Ossipov, D.A. Control of Growth Factor Binding and Release in Bisphosphonate Functionalized Hydrogels Guides Rapid Differentiation of Precursor Cells In Vitro. Biomater. Sci. 2016, 4, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Varghese, O.P.; Sun, W.; Hilborn, J.; Ossipov, D. In situ Cross-Linkable High Molecular Weight Hyaluronan-Bisphosphonate Conjugate for Localized Delivery and Cell-Specific Targeting: A Hydrogel Linked Prodrug Approach. J. Am. Chem. Soc. 2009, 131, 8781–8783. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, M.R.; Yang, X.; Bongio, M.; Alghamdi, H.; van den Beucken, J.; Huysmans, M.; Jansen, J.; Hilborn, J.; Ossipov, D.; Leeuwenburgh, S.C.G. Self-healing Hybrid Nanocomposites Consisting of Biosphosphonated Hyaluronan and Calcium Phosphate Nanoparticles. Biomaterials 2014, 35, 6918–6929. [Google Scholar] [CrossRef]
- Shi, L.; Carstensen, H.; Hölzl, K.; Lunzer, M.; Li, H.; Hilborn, J.; Ovsianikov, A.; Ossipov, D. Dynamic Coordination Chemistry Enables Free Directional Printing of Biopolymer Hydrogel. Chem. Mater. 2017, 29, 5816–5823. [Google Scholar] [CrossRef]
- Shi, L.; Han, Y.; Hilborn, J.; Ossipov, D. “Smart” drug loaded nanoparticle delivery from a self-healing hydrogel enabled by dynamic magnesium-biopolymer chemistry. Chem. Commun. 2016, 52, 11151–11154. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhao, Y.; Xie, Q.; Fan, C.; Hilborn, J.; Dai, J.; Ossipov, D. Moldable Hyaluronan Hydrogel Enabled by Dynamic Metal-Bisphosphonate Coordination Chemistry for Wound Healing. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; An, J.; Schmidt, S.; Hembury, M.; Ossipov, D.; Boccaccini, A.R.; Leeuwenburgh, S.C.G. Exploiting Bisphosphonate-Bioactive Glass Interactions for the Development of Self-Healing and Bioactive Composite Hydrogels. Macromol. Rapid Commun. 2016, 37, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Yang, X.; Shi, L.; Lanham, S.; Hilborn, J.; Oreffo, R.; Ossipov, D.; Dawson, J. Bisphosphonate nanoclay edge-site interactions facilitate hydrogel self-assembly and sustained growth factor localization. Nat. Commun. 2020, 11, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, K.; Hayashi, K.; Murata-Hirai, K.; Iwasaki, M.; Okamura, H.; Minato, N.; Morita, C.T.; Tanaka, Y. Targeting Cancer cells with Bisphosphonate Prodrug. Chem. Med. Chem. 2016, 11, 2656–2663. [Google Scholar] [CrossRef] [Green Version]
- Danial, M.; Telwatte, S.; Tyssen, D.; Cosson, S.; Tachedjian, G.; Moad, G.; Postma, A. Combination anti-HIV therapy via tandem release of prodrugs from macromolecular carriers. Polym. Chem. 2016, 7, 7477–7487. [Google Scholar] [CrossRef]
- Yu, T.; Zhuang, W.; Su, X.; Ma, B.; Hu, J.; He, H.; Li, G.; Wang, Y. Dual-Responsive Micelles with Aggregation-Induced Emission Feature and Two-Photon Absorption for Accurate Drug Delivery and Bioimaging. Bioconjugate Chem. 2019, 30, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Choi, K.Y.; Bhirde, A.; Swierczewska, M.; Yin, J.; Lee, S.W.; Park, J.H.; Hong, J.I.; Xie, J.; Niu, G.; et al. Sticky Nanoparticles: A Platform fore siRNA Delivery by a Bis(zinc(II)dipicolylamine)-Functionalized, Self-Assembled Nanoconjugate. Angew. Chem. Int. Ed. 2012, 51, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Su, J. Thiol-Mediated Chemoselective Strategies for In Situ Formation of Hydrogels. Gels 2018, 4, 72. [Google Scholar] [CrossRef] [Green Version]
- Gevrek, T.N.; Cosar, M.; Aydin, D.; Kaga, E.; Arslan, M.; Sanyal, R.; Sanyal, A. Facile Fabrication of a Modular ”Catch and Release” Hydrogel Iterface: Harnessing Thiol-Disulfide Exchange for Reversible Protein Capture and Cell Attachment. ACS Appl. Mater. Interfaces 2018, 10, 14399–14409. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, S.S.; Gonҫales, C.; Davis, L.; Gama, M. A novel Crosslinked Hyaluronic Acid Nanogel for Drug Delivery. Macromol. Biosci. 2014, 14, 1556–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, M.S.; Yang, D.H.; Lee, J.B.; Heo, D.N.; Kwon, Y.-D.; Youn, I.C.; Choi, K.; Hong, J.H.; Kim, G.T.; Choi, Y.S.; et al. Photo-cured hyaluronic acid-based hydrogels containing simvastatin as a tissue regeneration scaffold. Biomaterials 2011, 32, 8161–8171. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossipov, D.A.; Lüchow, M.; Malkoch, M. Differentiating Co-Delivery of Bisphosphonate and Simvastatin by Self-Healing Hyaluronan Hydrogel Formed by Orthogonal “Clicks”: An In-Vitro Assessment. Polymers 2021, 13, 2106. https://doi.org/10.3390/polym13132106
Ossipov DA, Lüchow M, Malkoch M. Differentiating Co-Delivery of Bisphosphonate and Simvastatin by Self-Healing Hyaluronan Hydrogel Formed by Orthogonal “Clicks”: An In-Vitro Assessment. Polymers. 2021; 13(13):2106. https://doi.org/10.3390/polym13132106
Chicago/Turabian StyleOssipov, Dmitri A., Mads Lüchow, and Michael Malkoch. 2021. "Differentiating Co-Delivery of Bisphosphonate and Simvastatin by Self-Healing Hyaluronan Hydrogel Formed by Orthogonal “Clicks”: An In-Vitro Assessment" Polymers 13, no. 13: 2106. https://doi.org/10.3390/polym13132106