Rheology and Water Absorption Properties of Alginate–Soy Protein Composites
Abstract
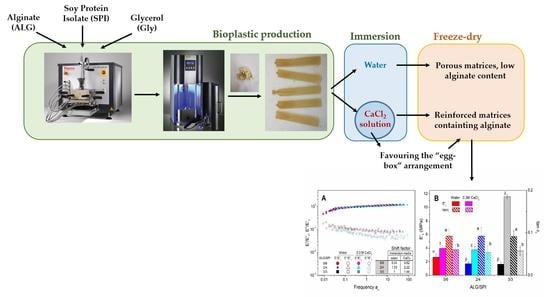
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methods
2.3.1. Dynamic Mechanical Analysis (DMA)
2.3.2. Tensile Tests until Break
2.3.3. Water Uptake Capacity
2.3.4. Statistical Analysis
3. Results
3.1. Mixing Stage
3.2. Rheological Characterization of Composite Blends
3.3. Rheological Characterization of Injection Moulded Composites
3.4. Mechanical Properties of Bioplastic Samples
3.5. Water Uptake Capacity and Soluble Matter Loss
3.6. Rheological Characterization of Freeze-Dried Matrices After Swelling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez-Puyana, V.; Felix, M.; Romero, A.; Guerrero, A. Development of pea protein-based bioplastics with antimicrobial properties. J. Sci. Food Agric. 2017, 97, 2671–2674. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Bengoechea, C.; Guerrero, A. Effect of pH on the properties of porcine plasma-based superabsorbent materials. Polym. Test. 2020, 85, 106453. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Khlestkin, V.K.; Peltek, S.E.; Kolchanov, N.A. Review of direct chemical and biochemical transformations of starch. Carbohydr. Polym. 2018, 181, 460–476. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Romero, A.; Bengoechea, C.; Guerrero, A. Natural superabsorbent plastic materials based on a functionalized soy protein. Polym. Test. 2017, 58, 126–134. [Google Scholar] [CrossRef]
- Cuadri, A.; Romero, A.; Bengoechea, C.; Guerrero, A. The Effect of Carboxyl Group Content on Water Uptake Capacity and Tensile Properties of Functionalized Soy Protein-Based Superabsorbent Plastics. J. Polym. Environ. 2018, 26, 2934–2944. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; del Toro, A.; Aguilar, J.M.; Bengoechea, C.; Guerrero, A. Optimization of a thermal process for the production of superabsorbent materials based on a soy protein isolate. Ind. Crops Prod. 2018, 125, 573–581. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Perez-Puyana, V.; Cordobés, F.; Romero, A.; Guerrero, A. Development of superabsorbent soy protein-based bioplastic matrices with incorporated zinc for horticulture. J. Sci. Food Agric. 2019, 99, 4825–4832. [Google Scholar] [CrossRef] [PubMed]
- Zohuriaan, J.; Kabiri, K. Superabsorbent Polymer Materials. A Review. Iran Polym J. 2008, 17, 451–477. [Google Scholar]
- Mallepally, R.R.; Bernard, I.; Marin, M.A.; Ward, K.R.; McHugh, M.A. Superabsorbent alginate aerogels. J. Supercrit. Fluids 2013, 79, 202–208. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Sengoku, K.; Fujioka, R. Pectin-based surperabsorbent hydrogels crosslinked by some chemicals: Synthesis and characterization. Polym. Bull. 2005, 55, 123–129. [Google Scholar] [CrossRef]
- Xu, M.; Qin, M.; Cheng, Y.; Niu, X.; Kong, J.; Zhang, X.; Huang, D.; Wang, H. Alginate microgels as delivery vehicles for cell-based therapies in tissue engineering and regenerative medicine. Carbohydr. Polym. 2021, 266, 118128. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Sa, B. 3-Polysaccharide-Based Superporous Hydrogels for Therapeutic Purposes; Maiti, S., Jana, S.B.T.-F.P., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 95–130. ISBN 978-0-08-102555-0. [Google Scholar]
- Du, L.; GhavamiNejad, A.; Yan, Z.-C.; Biswas, C.S.; Stadler, F.J. Effect of a functional polymer on the rheology and microstructure of sodium alginate. Carbohydr. Polym. 2018, 199, 58–67. [Google Scholar] [CrossRef]
- Teli, S.B.; Gokavi, G.S.; Aminabhavi, T.M. Novel sodium alginate-poly(N-isopropylacrylamide) semi-interpenetrating polymer network membranes for pervaporation separation of water+ethanol mixtures. Sep. Purif. Technol. 2007, 56, 150–157. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; Guo, N. Synthesis and properties of sodium alginate/Na+rectorite grafted acrylic acid composite superabsorbent via 60Coγ irradiation. Carbohydr. Polym. 2011, 85, 413–418. [Google Scholar] [CrossRef]
- Ma, X.; Li, R.; Zhao, X.; Ji, Q.; Xing, Y.; Sunarso, J.; Xia, Y. Biopolymer composite fibres composed of calcium alginate reinforced with nanocrystalline cellulose. Compos. Part A Appl. Sci. Manuf. 2017, 96, 155–163. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Chitosan–sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Çaykara, T.; Demirci, S.; Eroğlu, M.S.; Güven, O. Poly(ethylene oxide) and its blends with sodium alginate. Polymer 2005, 46, 10750–10757. [Google Scholar] [CrossRef]
- Mumper, R.J.; Huffman, A.S.; Puolakkainen, P.A.; Bouchard, L.S.; Gombotz, W.R. Calcium-alginate beads for the oral delivery of transforming growth factor-β1 (TGF-β1): Stabilization of TGF-β1 by the addition of polyacrylic acid within acid-treated beads. J. Control. Release 1994, 30, 241–251. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004. [Google Scholar] [CrossRef]
- Yeom, C.K.; Jegal, J.G.; Lee, K.H. Characterization of relaxation phenomena and permeation behaviors in sodium alginate membrane during pervaporation separation of ethanol–water mixture. J. Appl. Polym. Sci. 1996, 62, 1561–1576. [Google Scholar] [CrossRef]
- Orive, G.; Carcaboso, A.M.; Hernández, R.M.; Gascón, A.R.; Pedraz, J.L. Biocompatibility Evaluation of Different Alginates and Alginate-Based Microcapsules. Biomacromolecules 2005, 6, 927–931. [Google Scholar] [CrossRef]
- Orive, G.; Bartkowiak, A.; Lisiecki, S.; De Castro, M.; Hernández, R.M.; Gascòn, A.R.; Pedraz, J.L. Biocompatible oligochitosans as cationic modifiers of alginate/Ca microcapsules. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 429–439. [Google Scholar] [CrossRef]
- Reinhard, V.C.J.; Aaron, L.; Christopher, L.M.; Maree, H.T. Processability and mechanical properties of bioplastics produced from decoloured bloodmeal. Adv. Polym. Technol. 2017, 37, 2102–2113. [Google Scholar] [CrossRef]
- Delgado, M.; Felix, M.; Bengoechea, C. Development of bioplastic materials: From rapeseed oil industry by products to added-value biodegradable biocomposite materials. Ind. Crops Prod. 2018, 125, 401–407. [Google Scholar] [CrossRef]
- Martin-Alfonso, J.E.; Felix, M.; Romero, A.; Guerrero, A.; Martín-Alfonso, J.E.; Félix, M.; Romero, A.; Guerrero, A. Development of new albumen based biocomposites formulations by injection moulding using chitosan as physicochemical modifier additive. Compos. Part B Eng. 2014, 61, 275–281. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Ramos, M.; Bengoechea, C.; Martínez, I.; Romero, A. Effect of blend mixing and formulation on thermophysical properties of gluten-based plastics. J. Cereal Sci. 2020, 96, 103090. [Google Scholar] [CrossRef]
- Cho, S.-W.; Gällstedt, M.; Johansson, E.; Hedenqvist, M.S. Injection-molded nanocomposites and materials based on wheat gluten. Int. J. Biol. Macromol. 2011, 48, 146–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Castillo, E.; Bengoechea, C.; Guerrero, A. Strengthening of Porcine Plasma Protein Superabsorbent Materials through a Solubilization-Freeze-Drying Process. Polymers 2021, 13, 772. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Cordobés, F.; Guerrero, A. Thermomechanical properties and water uptake capacity of soy protein-based bioplastics processed by injection molding. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Perez, V.; Felix, M.; Romero, A.; Guerrero, A. Characterization of pea protein-based bioplastics processed by injection moulding. Food Bioprod. Process. 2016, 97, 100–108. [Google Scholar] [CrossRef]
- de Graaf, R.A.; Karman, A.P.; Janssen, L.P.B.M. Material properties and glass transition temperatures of different thermoplastic starches after extrusion processing. Starch-Stärke 2003, 55, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Castillo, E.; Bengoechea, C.; Rodríguez, N.; Guerrero, A. Development of green superabsorbent materials from a by-product of the meat industry. J. Clean. Prod. 2019, 223, 651–661. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Caballero, G.; Guerrero, A.; Bengoechea, C. Effect of Formulation and Pressure on Injection Moulded Soy Protein-Based Plastics. J. Polym. Environ. 2021. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Bengoechea, C.; Guerrero, A. Composites from by-products of the food industry for the development of superabsorbent biomaterials. Food Bioprod. Process. 2020, 119, 296–305. [Google Scholar] [CrossRef]
- Aguilar, J.M.; Bengoechea, C.; Pérez, E.; Guerrero, A. Effect of different polyols as plasticizers in soy based bioplastics. Ind. Crops Prod. 2020, 153, 112522. [Google Scholar] [CrossRef]
- Córdoba, A.; Cuéllar, N.; González, M.; Medina, J. The plasticizing effect of alginate on the thermoplastic starch/glycerin blends. Carbohydr. Polym. 2008, 73, 409–416. [Google Scholar] [CrossRef]
- Alonso-González, M.; Felix, M.; Romero, A. Development of malt sprout-based bioplastics via injection-moulding. Ind. Crops Prod. 2021, 162, 113267. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Sandía, J.A.A.; Cordobés, F.; Guerrero, A. Development of novel soy-protein-based superabsorbent matrixes through the addition of salts. J. Appl. Polym. Sci. 2019, 136, 47012. [Google Scholar] [CrossRef]
- Bengoechea, C.; Arrachid, A.; Guerrero, A.; Hill, S.E.S.E.; Mitchell, J.R.J.R. Relationship between the glass transition temperature and the melt flow behavior for gluten, casein and soya. J. Cereal Sci. 2007, 45, 275–284. [Google Scholar] [CrossRef]
- Félix, M.; Lucio-Villegas, A.; Romero, A.; Guerrero, A. Development of rice protein bio-based plastic materials processed by injection molding. Ind. Crops Prod. 2016, 79, 152–159. [Google Scholar] [CrossRef]
- Felix, M.; Perez-Puyana, V.; Romero, A.; Guerrero, A. Production and Characterization of Bioplastics Obtained by Injection Moulding of Various Protein Systems. J. Polym. Environ. 2017, 25, 91–100. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Avérous, L. Properties of glycerol-plasticized alginate films obtained by thermo-mechanical mixing. Food Hydrocoll. 2017, 63, 414–420. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Cordobés, F.; Guerrero, A. Protein/glycerol blends and injection-molded bioplastic matrices: Soybean versus egg albumen. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Oshodi, A.A.; Ojokan, E.-O. Effect of salts on some of the functional properties of bovine plasma protein concentrate. Food Chem. 1997, 59, 333–338. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and characterization of a novel super-absorbent based on wheat straw. Bioresour. Technol. 2011, 102, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Maltais, A.; Remondetto, G.E.; Gonzalez, R.; Subirade, M. Formation of soy protein isolate cold-set gels: Protein and salt effects. J. Food Sci. 2005, 70, C67–C73. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Pelagio, M.J.; Bengoechea, C.; Guerrero, A. Plasma based superabsorbent materials modulated through chemical cross-linking. J. Environ. Chem. Eng. 2021, 9, 105017. [Google Scholar] [CrossRef]








| (ALG + SPI)/Gly | ALG/SPI | wt% | ||
|---|---|---|---|---|
| SPI | ALG | Gly | ||
| 60/40 | 3/3 | 30 | 30 | 40 |
| 57/43 | 3/3 | 28.5 | 28.5 | 43 |
| 55/45 | 3/3 | 27.5 | 27.5 | 45 |
| 57/43 | 0/6 | 57 | 0 | 43 |
| 57/43 | 2/4 | 38 | 19 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Castillo, E.; Aguilar, J.M.; Bengoechea, C.; López-Castejón, M.L.; Guerrero, A. Rheology and Water Absorption Properties of Alginate–Soy Protein Composites. Polymers 2021, 13, 1807. https://doi.org/10.3390/polym13111807
Álvarez-Castillo E, Aguilar JM, Bengoechea C, López-Castejón ML, Guerrero A. Rheology and Water Absorption Properties of Alginate–Soy Protein Composites. Polymers. 2021; 13(11):1807. https://doi.org/10.3390/polym13111807
Chicago/Turabian StyleÁlvarez-Castillo, Estefanía, José Manuel Aguilar, Carlos Bengoechea, María Luisa López-Castejón, and Antonio Guerrero. 2021. "Rheology and Water Absorption Properties of Alginate–Soy Protein Composites" Polymers 13, no. 11: 1807. https://doi.org/10.3390/polym13111807