Copolymerization of Phthalic Anhydride with Epoxides Catalyzed by Amine-Bis(Phenolate) Chromium(III) Complexes
Abstract
:1. Introduction
2. Materials and Methods
General Copolymerization Procedure
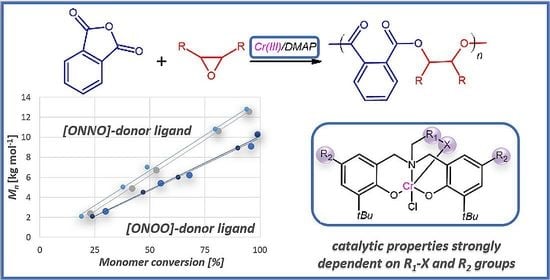
3. Results and Discussion
3.1. Co-Catalyst Effect
3.2. Ligand Structure Effect
3.3. Effect of Monomers to Catalytic System Ratio
3.4. Effect of Epoxide Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shyeni, P.; Yunqing, Z.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 2015, 51, 6459–6479. [Google Scholar] [CrossRef] [Green Version]
- Longo, J.M.; Sanford, M.J.; Coates, G.W. Ring-opening copolymerization of epoxides and cyclic anhydrides with discrete metal complexes: Structure–property relationships. Chem. Rev. 2016, 116, 15167–15197. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jia, J.; Xu, S.; Lao, K.U.; Sanford, M.J.; Ramakrishnan, R.K.; Nazarenko, S.I.; Hoye, T.R.; Coates, G.W.; DiStasio, R.A., Jr. Unraveling substituent effects on the glass transition temperatures of biorenewable polyesters. Nat. Commun. 2018, 9, 2880. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.F. Polyesters from expoxides and anhydrides. J. Polym. Sci. 1960, 44, 155–172. [Google Scholar] [CrossRef]
- Tsuruta, T.; Matsuura, K.; Inoue, S. Preparation of some polyesters by organometallic-catalyzed ring opening polymerization. Macromol. Chem. Phys. 1964, 75, 211–214. [Google Scholar] [CrossRef]
- Inoue, S.; Kitamura, K.; Tsuruta, T. Alternating copolymerization of phthalic anhydride and propylene oxide by dialkylzinc. Macromol. Chem. Phys. 1969, 126, 250–265. [Google Scholar] [CrossRef]
- Aida, T.; Inoue, S. Catalytic reaction on both sides of a metalloporphyrin plane. Alternating copolymerization of phthalic anhydride and epoxypropane with an aluminum porphyrin-quaternary salt system. J. Am. Chem. Soc. 1985, 107, 1358–1364. [Google Scholar] [CrossRef]
- Jeske, R.C.; DiCiccio, A.M.; Coates, G.W. Alternating copolymerization of epoxides and cyclic anhydrides: An improved route to aliphatic polyesters. J. Am. Chem. Soc. 2007, 129, 11330–11331. [Google Scholar] [CrossRef]
- Jeske, R.C.; Rowley, J.M.; Coates, G.W. Pre-rate-determining selectivity in the terpolymerization of epoxides, cyclic anhydrides, and CO2: A one-step route to diblock copolymers. Angew. Chem. Int. Ed. 2008, 47, 6041–6044. [Google Scholar] [CrossRef]
- DiCiccio, A.M.; Coates, G.W. Ring-opening copolymerization of maleic anhydride with epoxides: A chain-growth approach to unsaturated polyesters. J. Am. Chem. Soc. 2011, 133, 10724–10727. [Google Scholar] [CrossRef]
- Nejad, E.H.; Van Melis, C.G.W.; Vermeer, T.J.; Koning, C.E.; Duchateau, R. Alternating ring-opening polymerization of cyclohexene oxide and anhydrides: Effect of catalyst, cocatalyst, and anhydride structure. Macromolecules 2012, 45, 1770–1776. [Google Scholar] [CrossRef]
- Nejad, E.H.; Paoniasari, A.; Van Melis, C.G.W.; Koningand, C.E.; Duchateau, R. Catalytic ring-opening copolymerization of limonene oxide and phthalic anhydride: Toward partially renewable polyesters. Macromolecules 2013, 46, 631–637. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Poland, R.R.; Escobedo, C. Kinetic studies of the alternating copolymerization of cyclic acid anhydrides and epoxides, and the terpolymerization of cyclic acid anhydrides, epoxides, and CO2 catalyzed by (salen)CrIIICl. Macromolecules 2012, 45, 2242–2248. [Google Scholar] [CrossRef]
- Van Zee, N.J.; Coates, G.W. Alternating copolymerization of propylene oxide with biorenewable terpene-based cyclic anhydrides: A sustainable route to aliphatic polyesters with high glass transition temperatures. Angew. Chem. Int. Ed. 2015, 127, 2703–2706. [Google Scholar] [CrossRef]
- Huijser, S.; Nejad, E.H.; Sablong, R.; de Jong, C.; Koning, C.E.; Duchateau, R. Ring-opening co-and terpolymerization of an alicyclic oxirane with carboxylic acid anhydrides and CO2 in the presence of chromium porphyrinato and salen catalysts. Macromolecules 2011, 44, 1132–1139. [Google Scholar] [CrossRef]
- Robert, C.; de Montigny, F.; Thomas, C.M. Tandem synthesis of alternating polyesters from renewable resources. Nat. Commun. 2011, 2, 586. [Google Scholar] [CrossRef]
- Nejad, E.H.; Paoniasari, A.; Koning, C.E.; Duchateau, R. Semi-aromatic polyesters by alternating ring-opening copolymerisation of styrene oxide and anhydrides. Polym. Chem. 2012, 3, 1308–1313. [Google Scholar] [CrossRef]
- Duan, Z.; Wang, X.; Gao, Q.; Zhang, L.; Liu, B.; Kim, I. Highly active bifunctional cobalt-salen complexes for the synthesis of poly(ester-block-carbonate) copolymer via terpolymerization of carbon dioxide, propylene oxide, and norbornene anhydride isomer: Roles of anhydride conformation consideration. J. Polym. Sci. A Polym. Chem. 2014, 52, 789–795. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Eo, S.C.; Varghese, J.K.; Lee, B.Y. Copolymerization and terpolymerization of carbon dioxide/propylene oxide/phthalic anhydride using a (salen)Co(III) complex tethering four quaternary ammonium salts. Beilstein, J. Org. Chem. 2014, 10, 1787–1795. [Google Scholar] [CrossRef] [Green Version]
- Longo, J.M.; DiCiccio, A.M.; Coates, G.W. Poly(propylene succinate): A new polymer stereocomplex. J. Am. Chem. Soc. 2014, 136, 15897–15900. [Google Scholar] [CrossRef] [Green Version]
- Mundil, R.; Hošťálek, Z.; Šedenková, I.; Merna, J. Alternating ring-opening copolymerization of cyclohexene oxide with phthalic anhydride catalyzed by iron(III) salen complexes. Macromol. Res. 2015, 23, 161–166. [Google Scholar] [CrossRef]
- Proverbio, M.; Galotto Galotto, N.; Losio, S.; Tritto, I.; Boggioni, L. Influence of Co-catalysts and polymerization conditions on properties of poly(anhydride-alt-epoxide)s from rocop using salen complexes with different metals. Polymers 2019, 11, 1222. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.; Romain, C.; Meier, M.A.R.; Williams, C.K. Renewable polycarbonates and polyesters from 1,4-cyclohexadiene. Green Chem. 2015, 17, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.-F.; Zhu, L.-Q.; Wu, J.; Wu, L.-Y.; Lu, X.-Q. Ring-opening copolymerization of epoxides and anhydrides using manganese(III) asymmetrical Schiff base complexes as catalysts. RSC Adv. 2015, 5, 3854–3859. [Google Scholar] [CrossRef]
- Van Zee, N.J.; Sanford, M.J.; Coates, G.W. Electronic Effects of aluminum complexes in the copolymerization of propylene oxide with tricyclic anhydrides: Access to well-defined, Functionalizable Aliphatic Polyesters. J. Am. Chem. Soc. 2016, 138, 2755–2761. [Google Scholar] [CrossRef]
- DiCiccio, A.M.; Longo, J.M.; Rodríguez-Calero, G.G.; Coates, G.W. Development of highly active and regioselective catalysts for the copolymerization of epoxides with cyclic anhydrides: An unanticipated effect of electronic variation. J. Am. Chem. Soc. 2016, 138, 7107–7113. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ren, W.-M.; Lu, X.-B. Asymmetric alternating copolymerization of meso-epoxides and cyclic anhydrides: Efficient access to enantiopure polyesters. J. Am. Chem. Soc. 2016, 138, 11493–11496. [Google Scholar] [CrossRef]
- Fieser, M.E.; Sanford, M.J.; Mitchell, L.A.; Dunbar, C.R.; Mandal, M.; Van Zee, N.J.; Urness, D.M.; Cramer, C.J.; Coates, G.W.; Tolman, W.B. Mechanistic insights into the alternating copolymerization of epoxides and cyclic anhydrides using a (Salph)AlCl and iminium salt catalytic system. J. Am. Chem. Soc. 2017, 139, 15222–15231. [Google Scholar] [CrossRef]
- Hiranoi, Y.; Nakano, K. Copolymerization of epoxides with cyclic anhydrides catalyzed by dinuclear cobalt complexes. Beilstein J. Org. Chem. 2018, 14, 2779–2788. [Google Scholar] [CrossRef] [Green Version]
- Bester, K.; Bukowska, A.; Myśliwiec, B.; Hus, K.; Tomczyk, D.; Urbaniak, P.; Bukowski, W. Alternating ring-opening copolymerization of phthalic anhydride with epoxides catalysed by salophen chromium(III) complexes. An effect of substituents in salophen ligands. Polym. Chem. 2018, 9, 2147–2156. [Google Scholar] [CrossRef]
- Abel, B.A.; Lidston, C.A.L.; Coates, G.W. Mechanism-inspired design of bifunctional catalysts for the alternating ring-opening copolymerization of epoxides and cyclic anhydrides. J. Am. Chem. Soc. 2019, 141, 12760–12769. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Li, L.; Wen, Y.; Yanga, Q.; Duana, Z. Ring-opening copolymerization of epoxycyclohexane and phthalic anhydride catalyzed by the asymmetric Salen-CrCl complex. Polym. Int. 2020, 69, 513–518. [Google Scholar] [CrossRef]
- Harrold, N.D.; Li, Y.; Chisholm, M.H. Studies of ring-opening reactions of styrene oxide by chromium tetraphenylporphyrin initiators. Mechanistic and stereochemical considerations. Macromolecules 2013, 46, 692–698. [Google Scholar] [CrossRef]
- Robert, C.; Ohkawara, T.; Nozaki, K. Manganese-corrole complexes as versatile catalysts for the ring-opening homo-and co-polymerization of epoxide. Chem. Eur. J. 2014, 20, 4789–4795. [Google Scholar] [CrossRef]
- Si, G.; Zhang, L.; Han, B.; Duan, Z.; Liu, B.; Dong, J.; Li, X.; Liu, B. Novel chromium complexes with a [OSSO]-type bis(phenolato) dianionic ligand mediate the alternating ring-opening copolymerization of epoxides and phthalic anhydride. Polym. Chem. 2015, 6, 6372–6377. [Google Scholar] [CrossRef]
- Shaik, M.; Chidara, V.K.; Abbina, S.; Du, G. Zinc amido-oxazolinate catalyzed ring opening copolymerization and terpolymerization of maleic anhydride and epoxides. Molecules 2020, 25, 4044. [Google Scholar]
- Li, H.; Luo, H.; Zhao, J.; Zhang, G. Well-defined and structurally diverse aromatic alternating polyesters synthesized by simple phosphazene catalysis. Macromolecules 2018, 51, 2247–2257. [Google Scholar] [CrossRef]
- Dean, R.K.; Dawe, L.N.; Kozak, C.M. Copolymerization of cyclohexene oxide and co2 with a chromium diamine-bis(phenolate) catalyst. Inorg. Chem. 2012, 51, 9095–9103. [Google Scholar] [CrossRef]
- Dean, R.K.; Devaine-Pressing, K.; Dawe, L.N.; Kozak, C.M. Reaction of CO2 with propylene oxide and styrene oxide catalyzed by a chromium(III) amine-bis(phenolate) complex. Dalton Trans. 2013, 42, 9233–9244. [Google Scholar] [CrossRef]
- Chen, H.; Dawe, L.N.; Kozak, C.M. Chromium(III) amine-bis(phenolate) complexes as catalysts for copolymerization of cyclohexene oxide and CO2. Catal. Sci. Technol. 2014, 4, 1547–1555. [Google Scholar] [CrossRef]
- Devaine-Pressing, K.; Dawe, L.N.; Kozak, C.M. Cyclohexene oxide/carbon dioxide copolymerization by chromium(III) amino-bis(phenolato) complexes and MALDI-TOF MS analysis of the polycarbonates. Polym. Chem. 2015, 6, 6305–6315. [Google Scholar] [CrossRef] [Green Version]
- Kozak, C.M.; Woods, A.M.; Bottaro, C.S.; Devaine-Pressing, K.; Ni, K. A MALDI-TOF MS analysis study of the binding of 4-(N,N-dimethylamino)pyridine to amine-bis(phenolate) chromium(III) chloride complexes: Mechanistic insight into differences in catalytic activity for CO2/epoxide copolymerization. Faraday Discuss. 2015, 183, 31–46. [Google Scholar] [CrossRef]
- Ni, K.; Kozak, C.M. Kinetic studies of copolymerization of cyclohexene oxide with CO2 by a diamino-bis(phenolate) chromium(III) complex. Inorg. Chem. 2018, 57, 3097–3106. [Google Scholar] [CrossRef]
- Ni, K.; Paniez-Grave, V.; Kozak, C.M. Effect of azide and chloride binding to diamino-bis(phenolate) chromium complexes on CO2/cyclohexene oxide copolymerization. Organometallics 2018, 37, 2507–2518. [Google Scholar] [CrossRef]
- Dean, R.K.; Granville, S.L.; Dawe, L.N.; Decken, A.; Hattenhauera, K.M.; Kozak, C.M. Structure and magnetic behaviour of mono- and bimetallic chromium(III) complexes of amine-bis(phenolate) ligands. Dalton Trans. 2010, 39, 548–559. [Google Scholar] [CrossRef]
- Wichmann, O.; Sillanpää, R.; Lehtonen, A. Structural properties and applications of multidentate [O,N,O,X′] aminobisphenolate metal complexes. Coord. Chem. Rev. 2012, 256, 371–392. [Google Scholar] [CrossRef]
- Sekuła, J.; Nizioł, J.; Rode, W.; Ruman, T. Gold nanoparticle-enhanced target (AuNPET) as universal solution for laser desorption/ionization mass spectrometry analysis and imaging of low molecular weight compounds. Anal. Chim. Acta 2015, 875, 61–72. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, R.; Jia, L.; Fan, D.; Lü, X. Ring-opening copolymerization of CHO and MA catalyzed by the asymmetrical Cr(III)-bis-Schiff-base complex. Inorg. Chem. Commun. 2017, 79, 86–88. [Google Scholar] [CrossRef]
- Saini, P.K.; Romain, C.; Zhu, Y.; Williams, C.K. Di-magnesium and zinc catalysts for the copolymerization of phthalic anhydride and cyclohexene oxide. Polym. Chem. 2014, 5, 6068–6075. [Google Scholar] [CrossRef] [Green Version]
- Yu, Ch.-Y.; Chuang, H.-J.; Ko, B.-T. Bimetallic bis(benzotriazole iminophenolate) cobalt, nickel and zinc complexes as versatile catalysts for coupling of carbon dioxide with epoxides and copolymerization of phthalic anhydride with cyclohexene oxide. Catal. Sci. Technol. 2016, 6, 1779–1791. [Google Scholar] [CrossRef]
- Hošťálek, Z.; Trhlíkova, O.; Walterová, Z.; Martinez, T.; Peruch, F.; Cramail, H.; Merna, J. Alternating copolymerization of epoxides with anhydrides initiated by organic bases. Eur. Polym. J. 2017, 88, 433–447. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Mackiewicz, R.M.; Rodgers, J.L.; Fang, C.C.; Billodeaux, D.R.; Reibenspies, J.H. Cyclohexene oxide/CO2 copolymerization catalyzed by chromium(III) salen complexes and N-methylimidazole: Effects of varying salen ligand substituents and relative cocatalyst loading. Inorg. Chem. 2004, 43, 6024–6034. [Google Scholar] [CrossRef] [PubMed]








| Run | Catalytic System | PA Conversion d (%) | Ether e (mol%) | Mn f (kg mol−1) | Ð f | TON g | TOF h (h−1) |
|---|---|---|---|---|---|---|---|
| 1 a | – | 0 | – | – | – | – | – |
| 2 b | 1a | 2 | 66 | 0.8 | 1.44 | 5 | 5 |
| 3 c | PPh3 | 2 | – | – | – | 5 | 5 |
| 4 c | DBU | 5 | 65 | 1.1 | 1.06 | 12 | 12 |
| 5 c | BuImd | 11 | 63 | 2.9 | 1.10 | 28 | 28 |
| 6 c | DMAP | 14 | 57 | 3.8 | 1.08 | 35 | 35 |
| 7 | 1a/PPh3 | 21 | 14 | 2.6 | 1.22 | 52 | 52 |
| 8 | 1a/DBU | 26 | 13 | 3.3 | 1.19 | 65 | 65 |
| 9 | 1a/BuImd | 31 | 7 | 3.7 | 1.20 | 78 | 78 |
| 10 | 1a/DMAP | 42 | 6 | 4.8 | 1.18 | 105 | 105 |
| Run | Catalytic System | R1-X | R2 | t (min) | PA Conversion a (mol%) | Ether Units b (%) | Mn c (kg mol−1) | Ðc | TON d | TOF e (h−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a/DMAP | CH2NMe2 | tBu | 30 | 23 | 14 | 2.4 | 1.21 | 58 | 116 |
| 2 | 60 | 42 | 6 | 4.9 | 1.21 | 105 | 105 | |||
| 3 | 90 | 53 | 4 | 6.7 | 1.22 | 132 | 88 | |||
| 4 | 150 | 81 | 2 | 10.6 | 1.22 | 202 | 81 | |||
| 5 | 240 | 95 | 2 | 12.6 | 1.21 | 238 | 60 | |||
| 6 | 2a/DMAP | CH2NMe2 | OMe | 30 | 19 | 18 | 2.3 | 1.20 | 48 | 96 |
| 7 | 60 | 38 | 9 | 5.5 | 1.19 | 95 | 95 | |||
| 8 | 90 | 50 | 7 | 7.0 | 1.22 | 120 | 80 | |||
| 9 | 150 | 73 | 4 | 10.0 | 1.23 | 182 | 73 | |||
| 10 | 240 | 92 | 3 | 13.8 | 1.23 | 230 | 58 | |||
| 11 | 3a/DMAP | CH2NMe2 | F | 30 | 25 | 5 | 5.3 | 1.19 | 62 | 124 |
| 12 | 60 | 46 | 2 | 9.0 | 1.26 | 115 | 115 | |||
| 13 | 90 | 56 | 2 | 11.1 | 1.28 | 140 | 93 | |||
| 14 | 150 | 85 | 1 | 15.2 | 1.35 | 212 | 85 | |||
| 15 | 240 | >99 | <1 | 17.6 | 1.36 | 250 | 62 | |||
| 16 | 4a/DMAP | CH2OMe | tBu | 30 | 30 | 14 | 2.6 | 1.20 | 75 | 150 |
| 17 | 60 | 55 | 4 | 5.4 | 1.20 | 138 | 138 | |||
| 18 | 90 | 68 | 4 | 6.2 | 1.17 | 170 | 113 | |||
| 19 | 150 | 96 | 3 | 9.1 | 1.19 | 240 | 96 | |||
| 20 | 240 | >99 | 3 | 10.3 | 1.23 | 250 | 62 | |||
| 21 | 5a/DMAP | CH2OMe | OMe | 30 | 26 | 15 | 2.9 | 1.19 | 65 | 130 |
| 22 | 60 | 52 | 4 | 5.9 | 1.20 | 130 | 130 | |||
| 23 | 90 | 66 | 4 | 7.5 | 1.21 | 165 | 110 | |||
| 24 | 150 | 94 | 3 | 10.6 | 1.20 | 235 | 94 | |||
| 25 | 240 | >99 | 3 | 12.0 | 1.22 | 250 | 62 | |||
| 26 | 6a/DMAP | CH2OMe | F | 30 | 22 | 8 | 3.6 | 1.19 | 55 | 110 |
| 27 | 60 | 39 | 5 | 6.1 | 1.19 | 98 | 98 | |||
| 28 | 90 | 53 | 3 | 8.3 | 1.23 | 132 | 88 | |||
| 29 | 150 | 70 | 3 | 10.8 | 1.24 | 175 | 70 | |||
| 30 | 240 | 85 | 3 | 12.8 | 1.27 | 212 | 53 | |||
| 31 | 7a/DMAP |  | tBu | 30 | 19 | 14 | 2.1 | 1.19 | 48 | 96 |
| 32 | 60 | 38 | 5 | 5.0 | 1.19 | 95 | 95 | |||
| 33 | 90 | 49 | 5 | 7.0 | 1.20 | 122 | 81 | |||
| 34 | 150 | 80 | 2 | 10.8 | 1.21 | 200 | 80 | |||
| 35 | 240 | 94 | 2 | 12.8 | 1.24 | 235 | 59 | |||
| 36 | 8a/DMAP |  | tBu | 30 | 24 | 14 | 2.1 | 1.19 | 60 | 120 |
| 37 | 60 | 47 | 4 | 4.5 | 1.19 | 118 | 118 | |||
| 38 | 90 | 63 | 5 | 6.0 | 1.18 | 158 | 105 | |||
| 39 | 150 | 90 | 3 | 8.9 | 1.18 | 225 | 90 | |||
| 40 | 240 | 99 | 3 | 10.2 | 1.20 | 250 | 62 |
| Run | [PA]0:[CHO]0:[Cr]0:[DMAP]0 | t (min) | PA Conversion a (%) | Ether Units b (mol%) | Mn c (kg mol−1) | Ð c | TON g | TOF h (h−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 125:125:1:1 | 30 | 57 | 5 | 5.6 | 1.21 | 71 | 142 |
| 2 | 60 | 74 | 2 | 8.1 | 1.26 | 92 | 92 | |
| 3 | 90 | 90 | 1 | 9.3 | 1.27 | 112 | 75 | |
| 4 | 120 | >99 | <1 | 10.5 | 1.28 | 125 | 62 | |
| 5 | 250:250:1:1 | 30 | 25 | 5 | 5.3 | 1.19 | 62 | 124 |
| 6 | 60 | 46 | 2 | 9.0 | 1.26 | 115 | 115 | |
| 7 | 90 | 56 | 1 | 11.1 | 1.28 | 140 | 93 | |
| 8 | 150 | 85 | <1 | 15.2 | 1.35 | 212 | 85 | |
| 9 | 240 | >99 | <1 | 17.6 | 1.36 | 250 | 62 | |
| 10 | 500:500:1:1 | 30 | 10 | 11 | 2.6 | 1.20 | 50 | 100 |
| 11 | 60 | 16 | 5 | 4.2 | 1.22 | 80 | 80 | |
| 12 | 150 | 38 | 1 | 11.2 | 1.27 | 190 | 76 | |
| 13 | 240 | 57 | <1 | 14.8 | 1.30 | 285 | 71 | |
| 14 | 480 | 85 | <1 | 22.8 | 1.35 | 425 | 53 |
| Run | Epoxide | t (min) | PA Conversion a (%) | Ether Units b (mol%) | Mn c (kg mol−1) | Ð c | TON d | TOF e (h−1) | Tg f (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CHO | 60 | 46 | 2 | 9.0 | 1.26 | 115 | 115 | – |
| 2 | 240 | >99 | <1 | 17.6 | 1.36 | 250 | 62 | 141.8 | |
| 3 | 4-VCHO | 60 | 39 | 4 | 9.4 | 1.18 | 98 | 98 | – |
| 4 | 240 | 91 | <1 | 20.6 | 1.19 | 250 | 62 | 126.2 | |
| 5 | SO | 60 | 23 | 6 | 3.0 | 1.23 | 58 | 58 | – |
| 6 | 240 | 73 | 2 | 6.4 | 1.30 | 182 | 46 | 83.6 | |
| 7 | EFG | 60 | 38 | 3 | 10.6 | 1.17 | 95 | 95 | – |
| 8 | 240 | >99 | <1 | 17.9 | 1.68 | 250 | 62 | 57.6 | |
| 9 | PO | 60 | 62 | 1 | 12.3 | 1.15 | 155 | 155 | – |
| 10 | 240 | >99 | <1 | 17.0 | 1.27 | 250 | 62 | 59.6 | |
| 11 | BO | 60 | 34 | 3 | 8.0 | 1.18 | 85 | 85 | – |
| 12 | 240 | 98 | <1 | 17.7 | 1.18 | 245 | 61 | 47.7 | |
| 13 | ECH | 60 | 90 | <1 | 6.2 | 1.73 | 225 | 225 | – |
| 14 | 240 | >99 | <1 | 10.3 | 1.93 | 250 | 62 | 55.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukowski, W.; Bukowska, A.; Sobota, A.; Pytel, M.; Bester, K. Copolymerization of Phthalic Anhydride with Epoxides Catalyzed by Amine-Bis(Phenolate) Chromium(III) Complexes. Polymers 2021, 13, 1785. https://doi.org/10.3390/polym13111785
Bukowski W, Bukowska A, Sobota A, Pytel M, Bester K. Copolymerization of Phthalic Anhydride with Epoxides Catalyzed by Amine-Bis(Phenolate) Chromium(III) Complexes. Polymers. 2021; 13(11):1785. https://doi.org/10.3390/polym13111785
Chicago/Turabian StyleBukowski, Wiktor, Agnieszka Bukowska, Aleksandra Sobota, Maciej Pytel, and Karol Bester. 2021. "Copolymerization of Phthalic Anhydride with Epoxides Catalyzed by Amine-Bis(Phenolate) Chromium(III) Complexes" Polymers 13, no. 11: 1785. https://doi.org/10.3390/polym13111785