Cross-Linked Chitosan/Multi-Walled Carbon Nanotubes Composite as Ecofriendly Biocatalyst for Synthesis of Some Novel Benzil Bis-Thiazoles
Abstract
:1. Introduction
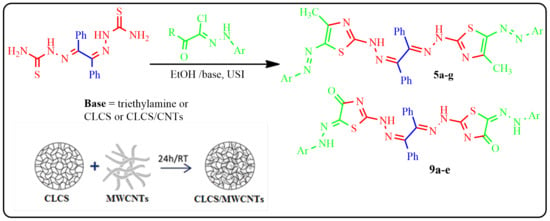
2. Results and Discussion
2.1. Preparation of CLCS and CLCS/MWCNTs Composite
2.2. FTIR Spectra of CLCS and CLCS/MWCNTs Composite
2.3. Scanning Electron Microscopy (SEM) of CLCS and CLCS/MWCNTs Composite
2.4. X-ray Diffraction of CLCS and CLCS/MWCNTs Composite
2.5. Thermal Stability of CLCS and CLCS/MWCNTs Composite
2.6. Synthesis of Benzil Bis-Thiazoles
3. Experimental Section
3.1. Measurements
3.2. Methods
3.2.1. Preparation of CLCS
3.2.2. Preparation of CLCS/MWCNTs Composite
3.2.3. Synthesis of Benzil Bis-Thiazole Derivatives 5a–g and 9a–e
3.2.4. Alternative Synthesis of 5a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molnár, Á. The use of chitosan-based metal catalysts in organic transformations. Coord. Chem. Rev. 2019, 388, 126–171. [Google Scholar] [CrossRef]
- Khalila, K.; Al-Matarb, H.; Elnagdib, M. Chitosan as an eco-friendly heterogeneous catalyst for Michael type addition reactions. A simple and efficient route to pyridones and phthalazines. Eur. J. Chem. 2010, 1, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Phan, N.T.S.; Le, K.K.A.; Nguyen, T.V.; Le, N.T.H. Chitosan as a renewable heterogeneous catalyst for the knoevenagel reaction in ionic liquid as green solvent. Isrn Org. Chem. 2012, 2012, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, N.A.; El-Ghany, N.A.A. Synthesis and antimicrobial activity of some novel terephthaloyl thiourea cross-linked carboxymethyl chitosan hydrogels. Cellulose 2012, 19, 1879. [Google Scholar] [CrossRef]
- Mohamed, N.A.; El-Ghany, N.A.A. Synthesis, characterization and antimicrobial activity of chitosan hydrazide derivative. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 410. [Google Scholar] [CrossRef]
- Lee, M.; Chen, B.-Y.; Den, W. Chitosan as a natural polymer for heterogeneous catalysts support: A short review on its applications. Appl. Sci. 2015, 5, 1272–1283. [Google Scholar] [CrossRef] [Green Version]
- Hasan, K.; Shehadi, I.A.; Al-Bab, N.D.; Elgamouz, A. Magnetic chitosan-supported silver nanoparticles: A heterogeneous catalyst for the reduction of 4-nitrophenol. Catalysts 2019, 9, 839. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.; Salam, A.; El-ziaty, A.K.; El-Sakhawy, M. New chitosan/silica/zinc oxide nanocomposite as adsorbent for dye removal. Inter. J. Biolog. Macromol. 2019, 131, 520–526. [Google Scholar] [CrossRef]
- Macquarrie, D.J.; Hardy, J.J.E. Applications of functionalized chitosan in catalysis. Ind. Eng. Chem. Res. 2005, 44, 8499–8520. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activity of arylazothiazoles and 1,3,4-thiadiazoles using chtosan-grafted-poly(4-vinylpyridine) as a novel copolymer basic catalyst. Chem. Heterocycl. Compd. 2015, 51, 1030–1038. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, N.A.; Mohamed, R.R.; El Latif, S.M.A. Chemically induced graft copolymerization of 4-vinyl pyridine onto carboxymethyl chitosan. Polym. Bull. 2011, 67, 693. [Google Scholar] [CrossRef]
- El-Harby, N.F.; Ibrahim, S.M.A.; Mohamed, N.A. Adsorption of Congo red dye onto antimicrobial terephthaloyl thiourea cross-linked chitosan hydrogels. Water Sci. Technol. 2017, 76, 2719. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Ganguly, J. Assessment of chitosan-based catalyst and their mode of action. Curr. Organocatal. 2019, 6, 106–138. [Google Scholar] [CrossRef]
- Dhanavel, S.; Manivannan, N.; Mathivanan, N.; Gupta, V.K.; Narayanan, V.; Stephen, A. Preparation and characterization of cross-linked chitosan/palladium nanocomposites for catalytic and antibacterial activity. J. Mol. Liq. 2018, 257, 32–41. [Google Scholar] [CrossRef]
- Jingjing, L.; Jiang, Z.C.P.; Leng, Y. Cross-linked chitosan supporting polyoxometalates catalyst with adjustable redox property for H2O2-based oxidation reactions. Catal. Commun. 2017, 94, 13–17. [Google Scholar]
- Shaabani, A.; Afshari, R.; Hooshmand, S.E. Crosslinked chitosan nanoparticle-anchored magnetic multi-wall carbon nanotubes: A bio-nanoreactor with extremely high activity toward click-multi-component reactions. N. J. Chem. 2017, 41, 8469–8481. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3]triazoles. Int. J. Biolog. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef]
- Mohamed, N.A.; El-Ghany, N.A.A. Synthesis, characterization and antimicrobial activity of novel aminosalicylhydrazide cross linked chitosan modified with multi-walled carbon nanotube. Cellulose 2019, 26, 1141. [Google Scholar] [CrossRef]
- El-Ghany, N.A.A. Antimicrobial activity of new carboxymethyl chitosan–carbon nanotube biocomposites and their swell ability in different pH media. J. Carbohydr. Chem. 2017, 36, 31–44. [Google Scholar] [CrossRef]
- Zarnegar, Z.; Safari, J. The novel synthesis of magnetically chitosan/carbon nanotube composites and their catalytic applications. Int. J. Biol. Macromol. 2015, 75, 21–31. [Google Scholar] [CrossRef]
- Limban, C.; Chifiriuc, M.C.B.; Missir, A.V.; Chiriţă, I.C.; Bleotu, C. Antimicrobial activity of some new thioureides derived from 2-(4-chlorophenoxymethyl)benzoic acid. Molecules 2008, 13, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Gomha, S.M.; Badrey, M.G.; Edrees, M.M. Heterocyclization of a bis-thiosemicarbazone of 2,5-diacetyl-3,4-disubstituted- thieno[2,3-b]thiophenebis- thiosemicarbazones leading to bis-thiazoles and bis-1,3,4-thiadiazoles as anti-breast cancer agents. J. Chem. Res. 2016, 40, 120–125. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; El-Reedy, A.A.M. Intramolecular ring transformation of bis-oxadiazoles to bis-thiadiazoles and investigation of their anticancer activities. J. Heterocycl. Chem. 2018, 55, 2360–2367. [Google Scholar] [CrossRef]
- Olar, R.; Badea, M.; Marinescu, D. N,N-dimethylbiguanide complexes displaying low cytotoxicity as potential large spectrum antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Limban, C.; Marutescu, L.; Chifiriuc, M.C. Synthesis, spectroscopic properties and antipathogenic activity of new thiourea derivatives. Molecules 2011, 16, 7593–7607. [Google Scholar] [CrossRef] [Green Version]
- John, M.; Beale, J.; Block, J.H. Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; Lippincott Williams & Wilkins, a Wolters Kluwer Business: Philadelphia, PA, USA, 2012; Volume 281–324, pp. 349–404. [Google Scholar]
- Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur. J. Med. Chem. 2009, 44, 1198–1204. [Google Scholar] [CrossRef]
- Mohamed, N.A.; El-Ghany, N.A.A. Novel aminohydrazide cross-linked chitosan filled with multi-walled carbon nanotubes as antimicrobial agents. Int. J. Biol. Macromol. 2018, 115, 651–662. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Sammour, M.H.; Elshafai, A.M. Novel self-dyed wholly aromatic polyamide-hydrazides covalently bonded with azo groups in their main chains: 2. Thermal characteristics. J. Therm. Anal. Calorim. 2013, 114, 859. [Google Scholar] [CrossRef]
- Mohamed, N.A.; El-Ghany, N.A.A.; Fahmy, M.M.; Khalaf-Alla, P.A. Novel polymaleimide containing dibenzoyl hydrazine pendant group as chelating agent for antimicrobial activity. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 68. [Google Scholar] [CrossRef]
- Gomha, S.M.; Edrees, M.M.; Altalbawy, F.M.A. Synthesis and characterization of some new bis-pyrazolyl-thiazoles incorporating the thiophene moiety as potent anti-tumor agents. Inter. J. Mol. Sci. 2016, 17, 1499. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelrazek, F.M.; Abdelrahman, A.H.; Metz, P. Synthesis of some novel thiazole, thiadiazole and 1,4-phenylene-bis-thiazole derivatives. Heterocycles 2016, 92, 954–967. [Google Scholar] [CrossRef] [Green Version]
- Abdelrazek, F.M.; Gomha, S.M.; Metz, P. Synthesis of some novel 1,4-phenylene-bis-thiazolyl derivatives and their anti-hypertensive α-blocking activity screening. J. Heterocycl. Chem. 2017, 54, 618–623. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Kassab, R.M.; Gomha, S.M. Synthesis and characterization of some novel bis-thiazoles. J. Heterocycl. Chem. 2019, 56, 3157–3163. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, H.M.; Matar, I.K.; El-Sayed, A.A. Green synthesis, molecular docking and anticancer activity of novel 1,4-dihydropyridine-3,5-dicarbohydrazones under grind-stone chemistry. Green Chem. Lett. Rev. 2020, 13, 6–17. [Google Scholar] [CrossRef]
- El-Enany, W.A.M.A.; Gomha, S.M.; Hussein, W.; Sallam, H.A.; Ali, R.S.; El-Ziaty, A.K. Synthesis and biological evaluation of some novel bis-thiadiazoles as antimicrobial and antitumor agents. Polycycl. Arom. Comp. 2020. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-aziz, H.M. Synthesis and antitumor activity of 1,3,4-thiadiazole derivatives bearing coumarine ring. Heterocycles 2015, 91, 583–592. [Google Scholar] [CrossRef]
- Abbas, I.M.; Riyadh, S.M.; Abdallah, M.A.; Gomha, S.M. A novel route to tetracyclic fused tetrazines and thiadiazines. J. Heterocycl. Chem. 2006, 43, 935–942. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-Aziz, H.A. Enaminones as building blocks in heterocyclic preparations: Synthesis of novel pyrazoles, pyrazolo[3,4-d]pyridazines, pyrazolo[1,5-a]pyrimidines, pyrido[2,3-d]pyrimidines linked to imidazo[2,1-b]thiazole system. Heterocycles 2012, 85, 2291–2303. [Google Scholar] [CrossRef]
- Yadav, R.C.; Sharma, P.K.; Singh, J. Synthesis and biological activity of 4″-substituted-2-(4′-formyl-3′- phenylpyrazole)-4-phenyl thiazole. J. Chem. Pharm. Res. 2013, 5, 78–84. [Google Scholar]








| Temperature (°C) | Weight Loss (%) | ||
|---|---|---|---|
| Chitosan | CLCS | CLCS/MWCNTs | |
| 50 | 2.24 | 0.48 | 0.72 |
| 100 | 6.36 | 1.92 | 3.62 |
| 150 | 8.61 | 4.79 | 6.75 |
| 200 | 8.98 | 7.42 | 8.44 |
| 250 | 10.47 | 10.05 | 11.33 |
| 260 | 11.22 | 11.72 | 12.54 |
| 270 | 13.09 | 15.55 | 14.47 |
| 280 | 15.33 | 22.26 | 17.60 |
| 290 | 19.08 | 29.20 | 21.95 |
| 300 | 25.06 | 34.94 | 27.48 |
| 310 | 34.04 | 37.81 | 32.55 |
| 320 | 40.76 | 39.97 | 35.68 |
| 330 | 43.76 | 41.64 | 37.86 |
| 340 | 46.01 | 43.07 | 39.79 |
| 350 | 47.87 | 44.51 | 41.23 |
| 400 | 53.86 | 48.93 | 46.53 |
| 450 | 59.09 | 53.20 | 50.15 |
| 500 | 65.46 | 57.95 | 53.29 |
| Compound no. | TEA | CLCS | CLCS/MWCNTs | |||
|---|---|---|---|---|---|---|
| Time (Min) | (%) Yield | Time (min) | (%) Yield | Time (min) | (%) Yield | |
| 5a | 50 | 77 | 41 | 79 | 20 | 85 |
| 5b | 50 | 74 | 43 | 80 | 23 | 86 |
| 5c | 56 | 78 | 46 | 79 | 25 | 86 |
| 5d | 53 | 72 | 43 | 80 | 28 | 84 |
| 5e | 52 | 72 | 36 | 81 | 19 | 87 |
| 5f | 60 | 78 | 38 | 83 | 25 | 85 |
| 5g | 55 | 73 | 38 | 82 | 22 | 87 |
| Compound no. | TEA | CLCS | CLCS/MWCNTs | |||
|---|---|---|---|---|---|---|
| Time (min) | (%) Yield | Time (min) | (%) Yield | Time (min) | (%) Yield | |
| 9a | 53 | 72 | 39 | 77 | 22 | 86 |
| 9b | 48 | 70 | 40 | 78 | 20 | 83 |
| 9c | 53 | 72 | 44 | 80 | 23 | 84 |
| 9d | 47 | 74 | 41 | 82 | 24 | 85 |
| 9e | 55 | 74 | 38 | 80 | 21 | 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshabanah, L.A.; Gomha, S.M.; Al-Mutabagani, L.A.; Abolibda, T.Z.; El-Ghany, N.A.A.; El-Enany, W.A.M.A.; El-Ziaty, A.K.; Ali, R.S.; Mohamed, N.A. Cross-Linked Chitosan/Multi-Walled Carbon Nanotubes Composite as Ecofriendly Biocatalyst for Synthesis of Some Novel Benzil Bis-Thiazoles. Polymers 2021, 13, 1728. https://doi.org/10.3390/polym13111728
Alshabanah LA, Gomha SM, Al-Mutabagani LA, Abolibda TZ, El-Ghany NAA, El-Enany WAMA, El-Ziaty AK, Ali RS, Mohamed NA. Cross-Linked Chitosan/Multi-Walled Carbon Nanotubes Composite as Ecofriendly Biocatalyst for Synthesis of Some Novel Benzil Bis-Thiazoles. Polymers. 2021; 13(11):1728. https://doi.org/10.3390/polym13111728
Chicago/Turabian StyleAlshabanah, Latifah A., Sobhi M. Gomha, Laila A. Al-Mutabagani, Tariq Z. Abolibda, Nahed A. Abd El-Ghany, Waleed A. M. A. El-Enany, Ahmed K. El-Ziaty, Rania S. Ali, and Nadia A. Mohamed. 2021. "Cross-Linked Chitosan/Multi-Walled Carbon Nanotubes Composite as Ecofriendly Biocatalyst for Synthesis of Some Novel Benzil Bis-Thiazoles" Polymers 13, no. 11: 1728. https://doi.org/10.3390/polym13111728