Synthesis of Optically Tunable and Thermally Stable PMMA–PVA/CuO NPs Hybrid Nanocomposite Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Copper Oxide Nanoparticles (CuO NPs)
2.3. Preparation of Copper Oxide Nanoparticles (CuO NPs)
3. Results and Discussion
3.1. UV-Vis Spectroscopy
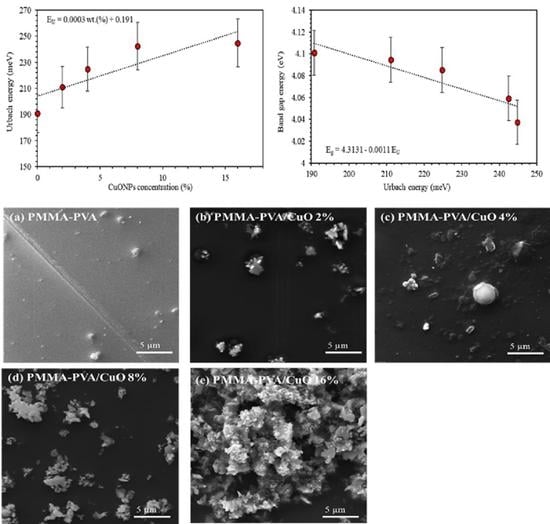
3.2. Optoelectronic Parameters
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Thermogravimetric Analysis (TGA)
3.5. Scanning Electron Microscope (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Gunaid, M.Q.; Saeed, A.M.; Subramani, N.K.; Madhukar, B. Optical parameters, electrical permittivity and I–V characteristics of PVA/Cs 2 CuO 2 nanocomposite films for opto-electronic applications. J. Mater. Sci. Mater. Electron. 2017, 28, 8074–8086. [Google Scholar] [CrossRef]
- Tao, P.; Li, Y.; Rungta, A.; Viswanath, A.; Gao, J.; Benicewicz, B.C.; Siegal, R.W.; Schadler, L.S. TiO 2 nanocomposites with high refractive index and transparency. J. Mater. Chem. 2011, 21, 18623–18629. [Google Scholar] [CrossRef]
- Liu, J.-G.; Ueda, M. High refractive index polymers: Fundamental research and practical applications. J. Mater. Chem. 2009, 19, 8907–8919. [Google Scholar] [CrossRef]
- Maeda, S.; Fujita, M.; Idota, N.; Matsukawa, K.; Sugahara, Y. Preparation of transparent bulk TiO2/PMMA hybrids with improved refractive indices via an in situ polymerization process using TiO2 nanoparticles bearing PMMA chains grown by surface-initiated atom transfer radical polymerization. ACS Appl. Mater. Interfaces 2016, 8, 34762–34769. [Google Scholar] [CrossRef]
- Mosley, D.W.; Auld, K.; Conner, D.; Gregory, J.; Liu, X.-Q.; Pedicini, A.; Thorsen, D.; Wills, M.; Khanarian, G.; Simon, E.S. High performance encapsulants for ultra high-brightness LEDs. In Light-Emitting Diodes: Research, Manufacturing, and Applications XII; Society of Photo Optical: Bellingham, WA, USA, 2008; p. 691017. [Google Scholar]
- Fujii, H.; Juni, N.; Tsutsumi, N. Enhanced coupling of light from organic electroluminescent device using diffusive particle dispersed high refractive index resin substrate. Opt. Rev. 2006, 13, 104–110. [Google Scholar]
- Krogman, K.C.; Druffel, T.; Sunkara, M.K. Anti-reflective optical coatings incorporating nanoparticles. Nanotechnology 2005, 16, S338. [Google Scholar] [CrossRef] [PubMed]
- Regolini, J.L.; Benoit, D.; Morin, P. Passivation issues in active pixel CMOS image sensors. Microelectron. Reliab. 2007, 47, 739–742. [Google Scholar] [CrossRef]
- Etefagh, R.; Rozati, S.; Azhir, E.; Shahtahmasebi, N.; Hosseini, A. Synthesis and antimicrobial properties of ZnO/PVA, CuO/PVA, and TiO2/PVA nanocomposites. Sci. Iran. 2017, 24, 1717–1723. [Google Scholar] [CrossRef] [Green Version]
- DeMerlis, C.; Schoneker, D. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Kadhum, R.; Hassen, T.; Abd alrasheed, N.F. Study of Gamma Irradiation Effect on the Optical Properties of Bromocresol Green Dye Doped Poly Methyl Methacrylate Thin Films. Results Phys. 2017, 7, 807–809. [Google Scholar]
- Demir, M.M.; Memesa, M.; Castignolles, P.; Wegner, G. PMMA/zinc oxide nanocomposites prepared by in-situ bulk polymerization. Macromol. Rapid Commun. 2006, 27, 763–770. [Google Scholar] [CrossRef] [Green Version]
- El Sayed, A.; El-Gamal, S.; Morsi, W.; Mohammed, G. Effect of PVA and copper oxide nanoparticles on the structural, optical, and electrical properties of carboxymethyl cellulose films. J. Mater. Sci. 2015, 50, 4717–4728. [Google Scholar] [CrossRef]
- Patake, V.; Joshi, S.; Lokhande, C.; Joo, O.-S. Electrodeposited porous and amorphous copper oxide film for application in supercapacitor. Mater. Chem. Phys. 2009, 114, 6–9. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, P.R. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Alsaad, A.; al Dairy, A.R.; Ahmad, A.; Al-anbar, A.S.; Al-Bataineh, Q.M. Synthesis and characterization of as-grown doped polymerized (PMMA-PVA)/ZnO NPs hybrid thin films. Polym. Bull. 2021, 1–22. [Google Scholar] [CrossRef]
- Alsaad, A.; al Dairy, A.R.; Ahmad, A.; Qattan, I.A.; al Fawares, S.; Al-Bataineh, Q. Synthesis and Characterization of Polymeric (PMMA-PVA) Hybrid Thin Films Doped with TiO2 Nanoparticles Using Dip-Coating Technique. Crystals 2021, 11, 99. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Ahmad, A.A.; Alsaad, A.; Telfah, A.D. Optical characterizations of PMMA/metal oxide nanoparticles thin films: Bandgap engineering using a novel derived model. Heliyon 2021, 7, e05952. [Google Scholar] [CrossRef] [PubMed]
- Alsaad, A.; Ahmad, A.; al Dairy, A.R.; Al-anbar, A.S.; Al-Bataineh, M.Q. Spectroscopic characterization of optical and thermal properties of (PMMA-PVA) hybrid thin films doped with SiO2 nanoparticles. Results Phys. 2020, 19, 103463. [Google Scholar] [CrossRef]
- Shariffudin, S.; Khalid, S.; Sahat, N.; Sarah, M.; Hashim, H. Preparation and characterization of nanostructured CuO thin films using sol-gel dip coating. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2015; p. 012007. [Google Scholar]
- Sonawane, R.; Hegde, S.; Dongare, M. Preparation of titanium (IV) oxide thin film photocatalyst by sol–gel dip coating. Mater. Chem. Phys. 2003, 77, 744–750. [Google Scholar] [CrossRef]
- Abouhaswa, A.; Taha, T. Tailoring the optical and dielectric properties of PVC/CuO nanocomposites. Polym. Bull. 2019, 1–12. [Google Scholar]
- Al-Hossainy, A.; Bassyouni, M.; Zoromba, M.S. Elucidation of electrical and optical parameters of poly (o-anthranilic acid)-poly (o-amino phenol)/copper oxide nanocomposites thin films. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2572–2583. [Google Scholar] [CrossRef]
- Fasasi, A.Y.; Osagie, E.; Pelemo, D.; Obiajunwa, E.; Ajenifuja, E.; Ajao, J.; Osinkolu, G.; Makinde, W.O.; Adeoye, A.E. Effect of Precursor Solvents on the Optical Properties of Copper Oxide Thin Films Deposited Using Spray Pyrolysis for Optoelectronic Applications. Am. J. Mater. Synth. Process. 2018, 3, 12–22. [Google Scholar]
- Moumen, A.; Hartiti, B.; Comini, E.; Arachchige, H.M.M.; Fadili, S.; Thevenin, P. Preparation and characterization of nanostructured CuO thin films using spray pyrolysis technique. Superlattices Microstruct. 2019, 127, 2–10. [Google Scholar] [CrossRef]
- Almusawe, A.J.; Kadhum, R.J.; Hassen, T.F.; Abd Alrasheed, N.F. Study of Gamma irradiation effect on the Optical Properties of Bromocresol Green Dye Doped Poly Methyl Methacrylate Thin Films. Eurasian J. Anal. Chem. 2018, 13, 152–158. [Google Scholar]
- Xu, B.; Zhou, J.; Ni, Z.; Zhang, C.; Lu, C. Synthesis of novel microencapsulated phase change materials with copper and copper oxide for solar energy storage and photo-thermal conversion. Sol. Energy Mater. Sol. Cells 2018, 179, 87–94. [Google Scholar] [CrossRef]
- Oubaha, M.; Elmaghrum, S.; Copperwhite, R.; Corcoran, B.; McDonagh, C.; Gorin, A. Optical Properties Of High Refractive Index Thin Films Processed At Low-Temperature. Opt. Mater. 2012, 34, 1366–1370. [Google Scholar] [CrossRef] [Green Version]
- Stuerga, D. Microwave-material interactions and dielectric properties, key ingredients for mastery of chemical microwave processes. Microw. Org. Synth. 2006, 2, 1–59. [Google Scholar]
- Ahmad, A.; Alsaad, A.; Al-Bataineh, Q.; Al-Naafa, M. Optical and structural investigations of dip-synthesized boron-doped ZnO-seeded platforms for ZnO nanostructures. Appl. Phys. A 2018, 124, 458. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Telfah, M.; Ahmad, A.A.; Alsaad, A.M.; Qattan, I.A.; Baaziz, H.; Charifi, Z.; Telfah, A. Synthesis, Crystallography, Microstructure, Crystal Defects, Optical and Optoelectronic Properties of ZnO: CeO2 Mixed Oxide Thin Films. Photonics 2020, 7, 112. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 6th ed.; Uno, Y., Tsuya, N., Morita, A., Yamashita, J., Eds.; Maruzen: Tokyo, Japan, 1986; pp. 124–129. [Google Scholar]
- Alsaad, A.; Al-Bataineh, Q.M.; Ahmad, A.; Albataineh, Z. A Telfah Optical band gap and refractive index dispersion parameters of boron-doped ZnO thin films: A novel derived mathematical model from the experimental transmission spectra. Optik 2020, 211, 164641. [Google Scholar] [CrossRef]
- Jin, Z.C.; Hamberg, I.; Granqvist, C. Optical properties of sputter-deposited ZnO: Al thin films. J. Appl. Phys. 1988, 64, 5117–5131. [Google Scholar] [CrossRef]
- Hassanien, A.S. Studies on dielectric properties, opto-electrical parameters and electronic polarizability of thermally evaporated amorphous Cd50S50− xSex thin films. J. Alloy. Compd. 2016, 671, 566–578. [Google Scholar] [CrossRef]
- Mol, B.; James, J.; Anoop, K.; Sulaniya, I.; Joseph, C.; Anantharaman, M.; Bushiri, J. Radio frequency plasma polymerized thin film based on eucalyptus oil as low dielectric permittivity, visible and near-infrared (NIR) photoluminescent material. J. Mater. Sci. Mater. Electron. 2019, 30, 12603–12611. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Alsaad, A.; Al-Bataineh, Q.M.; Bani-Salameh, A.A.; Ahmad, A.; Albiss, B.; Telfah, A.; Sabirianov, R.F. Synthesis and Structural, Crystallographic, Electronic, Chemical and Optical characterizations of Alpha-Diisopropylammonium Bromide (α-DIPAB) Thin Films. Optik 2021, 241, 167014. [Google Scholar] [CrossRef]
- Alsaad, A.; Al-Bataineh, Q.; Ahmad, A.; Bani-Salameh, A.; Albataineh, Z.; Telfah, A. Synthesis, crystallography, microstructure, crystal defects and optical properties of (fe-ni) co-doped zno thin films prepared by sol–gel technique. Bull. Am. Phys. Soc. 2020, 65. [Google Scholar]
- Ibrahim, S.M.; Bourezgui, A.; Al-Hossainy, A.F. Novel synthesis, DFT and investigation of the optical and electrical properties of carboxymethyl cellulose/thiobarbituric acid/copper oxide [CMC+ TBA/CuO] C nanocomposite film. J. Polym. Res. 2020, 27, 1–18. [Google Scholar] [CrossRef]
- El-Hagary, M.; Emam-Ismail, M.; Shaaban, E.; El-Taher, A. Effect of γ-irradiation exposure on optical properties of chalcogenide glasses Se70S30− xSbx thin films. Radiat. Phys. Chem. 2012, 81, 1572–1577. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Parmar, R.; Kundu, R.; Punia, R.; Aghamkar, P.; Kishore, N. Iron modified structural and optical spectral properties of bismuth silicate glasses. Phys. B: Condens. Matter 2014, 450, 39–44. [Google Scholar] [CrossRef]
- Chamroukhi, H.; Hamed, Z.B.; Telfah, A.; Bassou, M.; Zeinert, A.; Hergenröder, R.; Bouchriha, H. Optical and structural properties enhancement of hybrid nanocomposites thin films based on polyaniline doped with Zinc Oxide embedded in bimodal mesoporous silica (ZnO@ SiOX) nanoparticles. Opt. Mater. 2018, 84, 703–713. [Google Scholar] [CrossRef]
- Dhineshbabu, N.; Rajendran, V.; Nithyavathy, N.; Vetumperumal, R. Study of structural and optical properties of cupric oxide nanoparticles. Appl. Nanosci. 2016, 6, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Spitzer, W.; Fan, H. Determination of optical constants and carrier effective mass of semiconductors. Phys. Rev. 1957, 106, 882. [Google Scholar] [CrossRef]
- Alsaad, A.M.; Ahmad, A.A.; Al-Bataineh, Q.M.; Bani-Salameh, A.A.; Abdullah, H.S.; Qattan, I.A.; Albataineh, Z.H.; Telfah, A.H. Optical, Structural, and Crystal Defects Characterizations of Dip Synthesized (Fe-Ni) Co-Doped ZnO Thin Films. Materials 2020, 13, 1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farag, A.; Ashery, A.; Shenashen, M. Optical absorption and spectrophotometric studies on the optical constants and dielectric of poly (o-toluidine)(POT) films grown by spin coating deposition. Phys. B Condens. Matter 2012, 407, 2404–2411. [Google Scholar] [CrossRef]
- Ibupoto, Z.; Khun, K.; Beni, V.; Liu, X.; Willander, M. Synthesis of novel CuO nanosheets and their non-enzymatic glucose sensing applications. Sensors 2013, 13, 7926–7938. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, S. The Forensic Analysis of Skin-Safe. Stamp Pad Inks; John Jay College of Criminal Justice: New York, NY, USA, 2019. [Google Scholar]
- Kaur, S.; Samra, K.S. Study of Optical and Structural Properties of PVA/PMMA Blend; Lovely Professional University: Punjab, India, 2017. [Google Scholar]



















| CuO NPs (wt.%) | Refractive Index n | Extinction Constant k | Dielectric Permittivity | Dielectric Loss |
|---|---|---|---|---|
| 0 | 1.548 | 0.0077 | 2.396 | 0.0238 |
| 2 | 1.678 | 0.0224 | 2.814 | 0.0752 |
| 4 | 1.679 | 0.0291 | 2.816 | 0.0977 |
| 8 | 1.678 | 0.0381 | 2.814 | 0.1278 |
| 16 | 1.685 | 0.0417 | 2.836 | 0.1404 |
| Parameter | CuO 0% | CuO 2% | CuO 4% | CuO 8% | CuO 16% |
|---|---|---|---|---|---|
| Density of states, () | 1.066 | 1.116 | 0.944 | 0.691 | 0.812 |
| Charge carrier density, () | 4.273 | 4.472 | 3.782 | 2.770 | 3.255 |
| High frequency dielectric constant, | 2.623 | 3.058 | 3.017 | 2.948 | 3.003 |
| Relaxation time, (s) | 2.493 | 0.987 | 0.599 | 0.342 | 0.402 |
| Optical mobility, | 9.951 | 3.938 | 2.391 | 1.364 | 1.603 |
| Optical resistivity, | 1.470 | 3.548 | 6.910 | 16.548 | 11.979 |
| Parameter | PMMA–PVA | PMMA–PVA/TiO2 | PMMA–PVA/SiO2 | PMMA–PVA/CuO |
|---|---|---|---|---|
| Refractive index (at 550 nm) | 1.548 | 2.137 | 1.898 | 1.685 |
| Extenction coefficient (at 550 nm) | 0.0077 | 0.0181 | 0.0165 | 0.0417 |
| Optical Band gap (eV) | 4.101 | 4.050 | 4.047 | 4.037 |
() | 1.066 | 3.125 | 1.415 | 0.812 |
| High frequency dielectric constant, | 2.623 | 5.268 | 3.917 | 3.003 |
| 9.951 | 12.675 | 5.707 | 1.603 | |
| 1.470 | 0.394 | 1.932 | 11.979 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaad, A.M.; Ahmad, A.A.; Qattan, I.A.; El-Ali, A.-R.; Fawares, S.A.A.; Al-Bataineh, Q.M. Synthesis of Optically Tunable and Thermally Stable PMMA–PVA/CuO NPs Hybrid Nanocomposite Thin Films. Polymers 2021, 13, 1715. https://doi.org/10.3390/polym13111715
Alsaad AM, Ahmad AA, Qattan IA, El-Ali A-R, Fawares SAA, Al-Bataineh QM. Synthesis of Optically Tunable and Thermally Stable PMMA–PVA/CuO NPs Hybrid Nanocomposite Thin Films. Polymers. 2021; 13(11):1715. https://doi.org/10.3390/polym13111715
Chicago/Turabian StyleAlsaad, Ahmad M., Ahmad A. Ahmad, Issam A. Qattan, Abdul-Raouf El-Ali, Shatha A. Al Fawares, and Qais M. Al-Bataineh. 2021. "Synthesis of Optically Tunable and Thermally Stable PMMA–PVA/CuO NPs Hybrid Nanocomposite Thin Films" Polymers 13, no. 11: 1715. https://doi.org/10.3390/polym13111715