Enhanced Photocatalytic Activity of Cu2O Cabbage/RGO Nanocomposites under Visible Light Irradiation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. RGO/Cu2O Composite Synthesis
2.3. Characterization
2.4. Photocatalytic evaluation
3. Results and discussion
3.1. FT-IR Analysis
3.2. Structure Investigation
3.3. Raman analysis
3.4. UV–Vis Diffuse Reflectance Spectrum Studies
3.5. Thermogravimetric Analysis
3.6. FESEM and EDX Analysis
3.7. HR-TEM Analysis
3.8. EIS Analysis
3.9. Photocatalytic Studies
3.10. Kinetic Studies
- (i) CO and Ct—initial concentration and concentration of reactants at time ‘t’
- (ii) kabs and ‘t’—apparent rate constant and time, respectively.
3.11. Total Organic Carbon Analysis
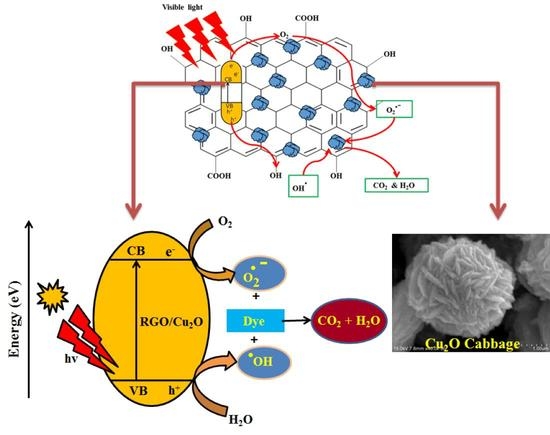
3.12. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Niu, H.; He, D.; Wang, S.; Cai, Y.; Zhang, Y. Hierarchical mesoporous carbon nanosheets for efficient organic pollutants removal. Mesoporous Mesoporous Mater. 2019, 276, 251–259. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, J.; Ma, J.; Shao, L. Highly regenerable alkali-resistant magnetic nanoparticles inspired by mussels for rapid selective dye removal offer high-efficiency environmental remediation. J. Mater. Chem. A 2015, 3, 19960–19968. [Google Scholar] [CrossRef]
- Ibrahim, D.S.; Anand, A.P.; Muthukrishnaraj, A.; Thilakavathi, R.; Balasubramanian, N. In situ electro-catalytic treatment of a reactive golden yellow HER synthetic dye effluent. J. Environ. Chem. Eng. 2013, 1, 2–8. [Google Scholar] [CrossRef]
- Zainith, S.; Sandhya, S.; Saxena, G.; Bharagava, R.N. Microbes an ecofriendly tools for the treatment of industrial waste waters. In Microbes and Environmental Management; Studium Press: New Delhi, Indian, 2016; pp. 78–103. [Google Scholar]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Sweleh, A.O. Optimizing textile dye removal by activated carbon prepared from olive stones. Environ. Technol. Innov. 2019, 16, 100488. [Google Scholar] [CrossRef]
- Mittal, H.; Maity, A.; Ray, S.S. Effective removal of cationic dyes from aqueous solution using gum ghatti-based biodegradable hydrogel. Int. J. Biol. Macromol. 2015, 79, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Karadag, D.; Akgul, E.; Tok, S.; Erturk, F.; Kaya, M.A.; Turan, M. Basic and Reactive Dye Removal Using Natural and Modified Zeolites. J. Chem. Eng. Data 2007, 52, 2436–2441. [Google Scholar] [CrossRef]
- Kadhom, M.; Albayati, N.; Alalwan, H.; Al-Furaiji, M. Removal of dyes by agricultural waste. Sustain. Chem. Pharm. 2020, 16, 100259. [Google Scholar] [CrossRef]
- Siyasukh, A.; Chimupala, Y.; Tonanon, N. Preparation of magnetic hierarchical porous carbon spheres with graphitic features for high methyl orange adsorption capacity. Carbon 2018, 134, 207–221. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, T.; He, Z. Enhanced Removal of Azo Dye by a Bioelectrochemical System Integrated with a Membrane Biofilm Reactor. Ind. Eng. Chem. Res. 2018, 57, 16433–16441. [Google Scholar] [CrossRef]
- Luo, J.; Fu, K.; Yu, D.; Hristovski, K.D.; Westerhoff, P.; Crittenden, J.C. Review of Advances in Engineering Nanomaterial Adsorbents for Metal Removal and Recovery from Water: Synthesis and Microstructure Impacts. ACS ES&T Eng. 2021, 1, 623–661. [Google Scholar] [CrossRef]
- Yang, T.; Yu, D.; Wang, D.; Yang, T.; Li, Z.; Wu, M.; Petru, M.; Crittenden, J. Accelerating Fe(III)/Fe(II) cycle via Fe(II) substitution for enhancing Fenton-like performance of Fe-MOFs. Appl. Catal. B Environ. 2021, 286, 119859. [Google Scholar] [CrossRef]
- Kariyajjanavar, P.; Jogttappa, N.; Nayaka, Y.A. Studies on degradation of reactive textile dyes solution by electrochemical method. J. Hazard. Mater. 2011, 190, 952–961. [Google Scholar] [CrossRef]
- Hsu, C.A.; Wen, T.N.; Su, Y.C.; Jiang, Z.B.; Chen, C.W.; Shyur, L.F. Biological Degradation of Anthroquinone and Azo Dyes by a Novel Laccase from Lentinus sp. Environ. Sci. Technol. 2012, 46, 5109–5117. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, L.; Wu, M.; Crittenden, J.C. Enhanced photocatalytic ozonation of organic pollutants using an iron-based metal-organic framework. Appl. Catal. B Environ. 2019, 251, 66–75. [Google Scholar] [CrossRef]
- Khan, S.; Narula, A.K. Synthesis of the ternary photocatalyst based on ZnO sensitized graphene quantum dot reinforced with conducting polymer exhibiting photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 6337–6349. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Shi, M.; Li, N.; Ma, H.; Ye, M. One Step Synthesis of Graphene Oxide−Magnetic Nanoparticle Composite. J. Phys. Chem. C 2010, 114, 1498–1503. [Google Scholar] [CrossRef]
- Sreeprasad, T.; Maliyekkal, S.M.; Lisha, K.; Pradeep, T. Reduced graphene oxide–metal/metal oxide composites: Facile synthesis and application in water purification. J. Hazard. Mater. 2011, 186, 921–931. [Google Scholar] [CrossRef]
- Xiang, H.; Tian, B.; Lian, P.; Li, Z.; Wang, H. Sol–gel synthesis and electrochemical performance of Li4Ti5O12/graphene composite anode for lithium-ion batteries. J. Alloy. Compd. 2011, 509, 7205–7209. [Google Scholar] [CrossRef]
- Muthukrishnaraj, A.; Vadivel, S.; Kamalakanan, V.P.; Balasubramanian, N.; Kamalakannan, V.P. α-Fe2O3/reduced graphene oxide nanorod as efficient photocatalyst for methylene blue degradation. Mater. Res. Innov. 2014, 19, 258–264. [Google Scholar] [CrossRef]
- Jin, Q.; Fujishima, M.; Nolan, M.; Anna, I.; Tada, H. Photocatalytic Activities of Tin(IV) Oxide Surface-Modified Titanium(IV) Dioxide Show a Strong Sensitivity to the TiO2 Crystal Form. J. Phys. Chem. C 2012, 116, 12621–12626. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Kondo, J.N.; Domen, K.; Shinohara, K.; Tanaka, A. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 357–358. [Google Scholar] [CrossRef]
- Sui, Y.; Fu, W.; Zeng, Y.; Yang, H.; Zhang, Y.; Chen, H.; Li, Y.; Li, M.; Zou, G. Synthesis of Cu2O Nanoframes and Nanocages by Selective Oxidative Etching at Room Temperature. Angew. Chem. Int. Ed. 2010, 49, 4282–4285. [Google Scholar] [CrossRef]
- Muthukrishnaraj, A.; Arun, A.; Kalaivani, S.; Maiyalagan, T.; Manikandan, A.; Balasubramanian, N. Solvothermal synthesis and characterizations of graphene-ZnBi12O20 nanocomposites for visible-light driven photocatalytic applications. Ceram. Int. 2020, 46, 18534–18543. [Google Scholar] [CrossRef]
- Luo, Y.; Li, S.; Ren, Q.; Liu, J.; Xing, L.; Wang, Y.; Yu, Y.; Jia, Z.; Li, J. Facile Synthesis of Flowerlike Cu2O Nanoarchitectures by a Solution Phase Route. Cryst. Growth Des. 2007, 7, 87–92. [Google Scholar] [CrossRef]
- Li, S.K.; Guo, X.; Wang, Y.; Huang, F.Z.; Shen, Y.H.; Wang, X.M.; Xie, A.J. Rapid synthesis of flower-like Cu2O architectures in ionic liquids by the assistance of microwave irradiation with high photochemical activity. Dalton Trans. 2011, 40, 6745–6750. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced Graphene Oxide Conjugated Cu2O Nanowire Mesocrystals for High-Performance NO2 Gas Sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Posa, V.R.; Annavaram, V.; Koduru, J.R.; AmmiReddy, V.R.; Somala, A.R. Graphene-ZnO nanocomposite for highly efficient photocatalytic degradation of methyl orange dye under solar light irradiation. Korean J. Chem. Eng. 2016, 33, 456–464. [Google Scholar] [CrossRef]
- Muthukrishnaraj, A.; Kalaivani, S.; Manikandan, A.; Kavitha, H.P.; Srinivasan, R.; Balasubramanian, N. Sonochemical synthesis and visible light induced photocatalytic property of reduced graphene oxide@ZnO hexagonal hollow rod nanocomposite. J. Alloy. Compd. 2020, 836, 155377. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. ZnO conjugated graphene: An efficient sunlight driven photocatalyst for degradation of organic dyes. Mater. Res. Bull. 2020, 129, 110911. [Google Scholar] [CrossRef]
- Ahmad, J.; Majid, K. Enhanced visible light driven photocatalytic activity of CdO-Graphene oxide heterostructures for the degredation of organic pollutants. New J. Chem. 2018, 42, 3246–3259. [Google Scholar] [CrossRef]
- Muthukrishnaraj, A.; Vadivel, S.; Joni, I.M.; Balasubramanian, N. Development of reduced graphene oxide/CuBi2O4 hybrid for enhanced photocatalytic behavior under visible light irradiation. Ceram. Int. 2015, 41, 6164–6168. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Liu, J.; Han, S.; Pan, Z.; Gan, L. High-efficient visible-light photocatalyst based on graphene incorporated Ag3PO4 nanocomposite applicable for the degradation of a wide variety of dyes. J. Photochem. Photobiol. A Chem. 2017, 340, 70–79. [Google Scholar] [CrossRef]
- Chnadel, N.; Dutta, V.; Sharma, S.; Raizada, P.; Sonu; Hosseini-Bandegharaei, A.; Kumar, R.; Singh, P.; Thakur, V. Z-scheme photocatalytic dye degradation on AgBr/Zn(Co)Fe2O4 photocatalysts supported on nitrogen-doped graphene. Mater. Today Sustain. 2020, 9, 100043. [Google Scholar] [CrossRef]
- Truong, N.T.; Thi, H.P.N.; Ninh, H.D.; Phung, X.T.; Van Tran, C.; Nguyen, T.T.; Pham, T.D.; Dang, T.D.; Chang, S.W.; Rene, E.R.; et al. Facile fabrication of graphene@Fe-Ti binary oxide nanocomposite from ilmenite ore: An effective photocatalyst for dye degradation under visible light irradiation. J. Water Process. Eng. 2020, 37, 101474. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M. Advanced nanostructured photocatalysts based on reduced graphene oxide–TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B Environ. 2012, 123–124, 241–256. [Google Scholar] [CrossRef]
- Kaushal, S.; Kaur, N.; Kaur, M.; Singh, P.P. Dual-Responsive Pectin/Graphene Oxide (Pc/GO) nano-composite as an efficient adsorbent for Cr (III) ions and photocatalyst for degradation of organic dyes in waste water. J. Photochem. Photobiol. A Chem. 2020, 403, 112841. [Google Scholar] [CrossRef]
- Wang, M.; Huang, J.; Tong, Z.; Li, W.; Chen, J. Reduced graphene oxide–cuprous oxide composite via facial deposition for photocatalytic dye-degradation. J. Alloy. Compd. 2013, 568, 26–35. [Google Scholar] [CrossRef]
- Han, F.; Li, H.; Yang, J.; Cai, X.; Fu, L. One-pot synthesis of cuprous oxide-reduced graphene oxide nanocomposite with enhanced photocatalytic and electrocatalytic performance. Phys. E Low-dimensional Syst. Nanostruct. 2016, 77, 122–126. [Google Scholar] [CrossRef]
- Nie, J.; Li, C.; Jin, Z.; Hu, W.; Wang, J.; Huang, T.; Wang, Y. Fabrication of MCC/Cu2O/GO composite foam with high photocatalytic degradation ability toward methylene blue. Carbohydr. Polym. 2019, 223, 115101. [Google Scholar] [CrossRef]
- Pu, Y.; Chou, H.; Kuo, W.; Wei, K.; Hsu, Y. Interfacial charge carrier dynamics of cuprous oxide-reduced graphene oxide (Cu2O-rGO) nanoheterostructures and their related visible-light-driven photocatalysis. Appl. Catal. B Environ. 2017, 204, 21–32. [Google Scholar] [CrossRef]
- Hasijaa, V.; Raizadaab, P.; Sudhaika, A.; Singhab, P.; Thakur, V.K.; Khan, A.A.P. Fabrication of Ag/AgI/WO3 heterojunction anchored P and S co-doped graphitic carbon nitride as a dual Z scheme photocatalyst for efficient dye degradation. Solid State Sci. 2020, 100, 106095. [Google Scholar] [CrossRef]
- Chandel, N.; Sharma, K.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Singh, P. Magnetically separable ZnO/ZnFe2O4 and ZnO/CoFe2O4 photocatalysts supported onto nitrogen doped graphene for photocatalytic degradation of toxic dyes. Arab. J. Chem. 2020, 13, 4324–4340. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, J.; Kim, J. Photocatalytic Activity and Filtration Performance of Hybrid TiO2-Cellulose Acetate Nanofibers for Air Filter Applications. Polym. 2021, 13, 1331. [Google Scholar] [CrossRef] [PubMed]















| S. No. | Samples | % Degradation | |
|---|---|---|---|
| MB | MO | ||
| 1 | Blank | 8.29 | 5.88 |
| 2 | Degussa P25 | 16.41 | 19.87 |
| 3 | Cu2O | 59.44 | 47.82 |
| 4 | GCC 1 | 77.7 | 77.84 |
| 5 | GCC 2 | 97.9 | 96.1 |
| 6 | GCC 3 | 90.18 | 85.68 |
| 7 | GCC 4 | 85.65 | 80.13 |
| Types of Graphene Metal Oxides | Methylene Blue (%) | Methyl Orange (%) | References |
|---|---|---|---|
| GO/ZnO | - | 97 | [30] |
| RGO/ZnO | 90.72 | 89.72 | [31] |
| GO/ZnO | 100 | 100 | [32] |
| GO/CdO | 97 | 89 | [33] |
| RGO/CuBi2O4 | 95 | 87 | [34] |
| RGO/ZnBi12O20 | 96.04 | 94.52 | [26] |
| GO/Ag3PO4 | 100 | 100 | [35] |
| *NG/AgBr/CF | – | 98 | [36] |
| GO/Fe-Ti | 100 | – | [37] |
| GO/TiO2 | – | 100 | [38] |
| GO/Pectin | 98 | 87.5 | [39] |
| rGO/Cu2O/PA | – | 95 | [40] |
| rGO/Cu2O | 95 | – | [41] |
| GO/MCC/Cu2O | 83.62 | – | [42] |
| RGO/Cu2O | – | 98 | [43] |
| RGO/Cu2O | 97.9 | 96.1 | Present work |
| Samples | kabs (min−1) | ||||||
|---|---|---|---|---|---|---|---|
| Blank | Degussa P25 | Cu2O | GCC 1 | GCC 2 | GCC 3 | GCC 4 | |
| MB | 0 | 0 | 8 × 10−3 | 16 × 10−3 | 58 × 10−3 | 22 × 10−3 | 16 × 10−3 |
| MO | 0 | 1 × 10-3 | 3 × 10−3 | 5 × 10−3 | 5 × 10−3 | 6 × 10−3 | 7 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthukrishnaraj, A.; Al-Zahrani, S.A.; Al Otaibi, A.; Kalaivani, S.S.; Manikandan, A.; Balasubramanian, N.; Bilgrami, A.L.; Ahamed, M.A.R.; Khan, A.; Asiri, A.M.; et al. Enhanced Photocatalytic Activity of Cu2O Cabbage/RGO Nanocomposites under Visible Light Irradiation. Polymers 2021, 13, 1712. https://doi.org/10.3390/polym13111712
Muthukrishnaraj A, Al-Zahrani SA, Al Otaibi A, Kalaivani SS, Manikandan A, Balasubramanian N, Bilgrami AL, Ahamed MAR, Khan A, Asiri AM, et al. Enhanced Photocatalytic Activity of Cu2O Cabbage/RGO Nanocomposites under Visible Light Irradiation. Polymers. 2021; 13(11):1712. https://doi.org/10.3390/polym13111712
Chicago/Turabian StyleMuthukrishnaraj, Appusamy, Salma Ahmed Al-Zahrani, Ahmed Al Otaibi, Semmedu Selvaraj Kalaivani, Ayyar Manikandan, Natarajan Balasubramanian, Anwar L. Bilgrami, Mohamed A. Riswan Ahamed, Anish Khan, Abdulaah M. Asiri, and et al. 2021. "Enhanced Photocatalytic Activity of Cu2O Cabbage/RGO Nanocomposites under Visible Light Irradiation" Polymers 13, no. 11: 1712. https://doi.org/10.3390/polym13111712