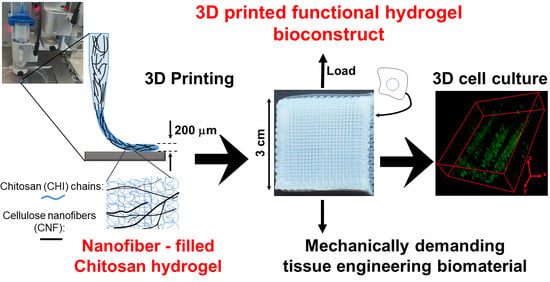
Development of Bioinspired Functional Chitosan/Cellulose Nanofiber 3D Hydrogel Constructs by 3D Printing for Application in the Engineering of Mechanically Demanding Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chitosan Source and Characterization
2.2. Cellulose Nanofibers
2.3. Transmission Electron Microscopy
2.4. Preparation of Chitosan/Cellulose Nanofiber Viscous Inks
Rheological Behavior
2.5. 3D Printing of Cellulose Nanofiber-Filled Chitosan Hydrogel Scaffolds
2.5.1. Scanning Electron Microscopy
2.5.2. Microtensile Testing
2.5.3. Wide and Small-Angle X-ray Synchrotron Scattering (WAXS and SAXS). In Situ Microtensile Testing
2.6. 3D Cell Culture of Fibroblasts in the Printed Hydrogel Scaffolds
Live/Dead Cell Viability Assay
3. Results and Discussion
3.1. Cellulose Nanofibers Microstructure
3.2. Chitosan/Cellulose Nanofiber Inks Rheological Behavior
3.3. Cellulose Nanofiber-Filled Chitosan Printed Hydrogels
3.3.1. Morphology and Dimensions
3.3.2. Mechanical Properties
3.3.3. Synchrotron X-ray Scattering SAXS/WAXS Analyses of Printed Hydrogels. Characterization of the Cellulose Nanofibers Dispersion and Orientation
3.3.4. Experimental Design for the Optimization of the Size Resolution and Mechanical Performance of CHI/CNF Printed Hydrogels
3.4. 3D Printed CHI/CNF Hydrogel Scaffolds
Suitability of 3D Printed CHI/CNF Hydrogel Scaffolds for Three-Dimensional Cell Culture
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gensler, M.; Leikeim, A.; Möllmann, M.; Komma, M.; Heid, S.; Müller, C.; Boccaccini, A.R.; Salehi, S.; Groeber-Becker, F.; Hansmann, J. 3D printing of bioreactors in tissue engineering: A generalised approach. PLoS ONE 2020, 15, e0242615. [Google Scholar] [CrossRef] [PubMed]
- Parra-Cantu, C.; Li, W.; Quiñones-Hinojosa, A.; Zhang, Y.S. 3D bioprinting of glioblastoma models. J. 3D Print. Med. 2020, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.; Jiang, N.; Parra-Cantu, C.; Tang, G.; Zhang, J.; Wang, H.; Chen, S.; Huang, N.; Xie, J.; Zhang, Y.S. Bioprinted Injectable Hierarchically Porous Gelatin Methacryloyl Hydrogel Constructs with Shape-Memory Properties. Adv. Funct. Mater. 2020, 30, 2003740. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Li, W.; Cao, X.; Wang, Z.; Wang, M.; Robledo-Lara, J.A.; Liao, J.; Chávez-Madero, C.; Hassan, S.; et al. Expanding sacrificially printed microfluidic channel-embedded paper devices for construction of volumetric tissue models in vitro. Biofabrication 2020, 12, 045027. [Google Scholar] [CrossRef] [PubMed]
- Apsite, I.; Constante, G.; Dulle, M.; Vogt, L.; Caspari, A.; Boccaccini, A.R.; Synytska, A.; Salehi, S.; Ionov, L. 4D Biofabrication of fibrous artificial nerve graft for neuron regeneration. Biofabrication 2020, 12, 035027. [Google Scholar] [CrossRef]
- Heid, S.; Boccaccini, A.R. Advancing bioinks for 3D bioprinting using reactive fillers: A review. Acta Biomater. 2020, 113, 1–22. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Hung, K.-C.; Chen, C.-W. Biodegradable polymer scaffolds. J. Mater. Chem. B 2016, 4, 7493–7505. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazur, J.; Detsch, R.; Karakaya, E.; Kaschta, J.; Tessmar, J.; Schneidereit, D.; Friedrich, O.; Schubert, D.W.; Boccaccini, A.R. Improving alginate printability for biofabrication: Establishment of a universal and homogeneous pre-crosslinking technique. Biofabrication 2020, 12, 045004. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.J.; Chung, S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; A Park, S. Cell-laden 3D bioprinting hydrogel matrix depending on different compositions for soft tissue engineering: Characterization and evaluation. Mater. Sci. Eng. C 2017, 71, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Devarasetty, M.; Kang, H.-W.; Mead, I.; Bishop, C.; Shupe, T.; Lee, S.J.; Jackson, J.; Yoo, J.; Soker, S.; et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015, 25, 24–34. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Hauptstein, J.; Böck, T.; Bartolf-Kopp, M.; Forster, L.; Stahlhut, P.; Nadernezhad, A.; Blahetek, G.; Zernecke-Madsen, A.; Detsch, R.; Jüngst, T.; et al. Hyaluronic Acid-Based Bioink Composition Enabling 3D Bioprinting and improving quality of deposited cartilaginous extracellular matrix. Adv. Health Mater. 2020, 9, 2000737. [Google Scholar] [CrossRef] [PubMed]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, H.; Bao, G.; Luo, Z.; Mongeau, L.G.; Zhang, Y.S. Composite inks for extrusion printing of biological and biomedical constructs. ACS Biomater. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, Z.; Hu, N.; Zhou, Y.; Maggio, L.; Miri, A.K.; Fragasso, A.; Jin, X.; Khademhosseini, A.; Zhang, Y.S. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 2017, 10, 024102. [Google Scholar] [CrossRef] [PubMed]
- Chedly, J.; Soares, S.; Montembault, A.; von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.-N.; Taxi, J.; David, L.; Nothias, F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 2017, 138, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Doench, I.; Tran, T.A.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N.; et al. Cellulose nanofiber-reinforced chitosan hydrogel composites for intervertebral disc tissue repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; De Oliveira, P.N.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.S.M.; Osorio-Madrazo, A. Injectable and gellable chitosan formulations filled with cellulose nanofibers for intervertebral disc tissue engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiamingo, A.; Montembault, A.; Boitard, S.-E.; Naemetalla, H.; Agbulut, O.; Delair, T.; Campana-Filho, S.P.; Menasché, P.; David, L. Chitosan hydrogels for the regeneration of infarcted myocardium: Preparation, physicochemical characterization, and biological evaluation. Biomacromolecules 2016, 17, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Rami, L.; Malaise, S.; Delmond, S.; Fricain, J.-C.; Siadous, R.; Schlaubitz, S.; Laurichesse, E.; Amedee, J.; Montembault, A.; David, L.; et al. Physicochemical modulation of chitosan-based hydrogels induces different biological responses: Interest for tissue engineering. J. Biomed. Mater. Res. Part A 2013, 102, 3666–3676. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.; Tahiri, K.; Montembault, A.; Domard, A.; Corvol, M.-T. Multi-membrane chitosan hydrogels as chondrocytic cell bioreactors. Biomaterials 2011, 32, 5354–5364. [Google Scholar] [CrossRef]
- De Souza, R.F.B.; De Souza, F.C.B.; Rodrigues, C.; Drouin, B.; Popat, K.C.; Mantovani, D.; Moraes, Â.M. Mechanically-enhanced polysaccharide-based scaffolds for tissue engineering of soft tissues. Mater. Sci. Eng. C 2019, 94, 364–375. [Google Scholar] [CrossRef]
- García, D.E.; Glasser, W.G.; Pizzi, T.A.; Osorio-Madrazo, A.; Laborie, M.-P.G. Synthesis and physicochemical properties of hydroxypropyl tannins from maritime pine bark (Pinus pinaster Ait.). Holzforschung 2014, 68, 411–418. [Google Scholar] [CrossRef]
- Osorio Madrazo, A. Whiskers of chitosan for bio-nano-composites. Ph.D. Thesis, Laboratoire Ingénierie des Matériaux Polymères IMP, Claude Bernard University Lyon 1, Villeurbanne, France, 2008. [Google Scholar]
- Agüero, H.P.; David, L.; Covas, C.P.; Osorio-Madrazo, A. Bioinspired chitosan-BSA fibers for applications in tissue engineering of the fibrous ring of intervertebral discs. Rev. Cuba. Investig. Bioméd. 2017, 36, 1–11. [Google Scholar]
- Peniche, H.; Osorio, A.; Acosta, N.; De La Campa, A.; Peniche, C. Preparation and characterization of superparamagnetic chitosan microspheres: Application as a support for the immobilization of tyrosinase. J. Appl. Polym. Sci. 2005, 98, 651–657. [Google Scholar] [CrossRef]
- Toeri, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation and chemical/microstructural characterization of azacrown ether-crosslinked chitosan films. Materials 2017, 10, 400. [Google Scholar] [CrossRef]
- Von Palubitzki, L.; Wang, Y.; Hoffmann, S.; Vidal-Y-Sy, S.; Zobiak, B.; Failla, A.V.; Schmage, P.; John, A.; Osorio-Madrazo, A.; Bauer, A.T.; et al. Differences of the tumour cell glycocalyx affect binding of capsaicin-loaded chitosan nanocapsules. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Osorio, A.; Trombotto, S.; Lucas, J.-M.; Peniche, C.; David, L.; Domard, A. Solid-state acid hydrolysis of chitosan: Evolution of the crystallinity and the macromolecular structure. In Proceedings of the 10th International Conference on Chitin and Chitosan, 7th International Conference of the European Chitin Society, Montpellier, France, 6–9 September 2006; pp. 609–615. [Google Scholar]
- Gorzelanny, C.; Pöppelmann, B.; Pappelbaum, K.; Moerschbacher, B.M.; Schneider, S.W. Human macrophage activation triggered by chitotriosidase-mediated chitin and chitosan degradation. Biomaterials 2010, 31, 8556–8563. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Gupta, P.K.; Bhonde, R.; Totey, S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Prolif. 2011, 44, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Montembault, A.; Tahiri, K.; Korwin-Zmijowska, C.; Chevalier, X.; Corvol, M.-T.; Domard, A. A material decoy of biological media based on chitosan physical hydrogels: Application to cartilage tissue engineering. Biochimie. 2006, 88, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Madrazo, A.; Eder, M.; Rueggeberg, M.; Pandey, J.K.; Harrington, M.J.; Nishiyama, Y.; Putaux, J.-L.; Rochas, C.; Burgert, I. Reorientation of cellulose nanowhiskers in agarose hydrogels under tensile loading. Biomacromolecules 2012, 13, 850–856. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Covas, C.A.P.; Rochas, C.; Putaux, J.-L.; Trombotto, S.; Alcouffe, P.; Domard, A. Fine microstructure of processed chitosan nanofibril networks preserving directional packing and high molecular weight. Carbohydr. Polym. 2015, 131, 1–8. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Covas, C.A.P.; Domard, A. Highly crystalline chitosan produced by multi-steps acid hydrolysis in the solid-state. Carbohydr. Polym. 2011, 83, 1730–1739. [Google Scholar] [CrossRef]
- Samyn, P.; Osorio-Madrazo, A. Native crystalline polysaccharide nanofibers: Processing and properties. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Germany, 2018; pp. 1–36. [Google Scholar]
- Osorio-Madrazo, A.; Laborie, M.P. Morphological and thermal investigations of cellulosic bionanocomposites. In Biopolymer Nanocomposites; Dufresne, A., Thomas, S., Pothen, L.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 411–435. [Google Scholar]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation of cellulose I nanowhiskers with a mildly acidic aqueous ionic liquid: Reaction efficiency and whiskers attributes. Cellulose 2013, 20, 1829–1840. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaille, J.Y. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Marquez-Bravo, S.; Doench, I.; Molina, P.; Bentley, F.; Tamo, A.; Passieux, R.; Lossada, F.; David, L.; Osorio-Madrazo, A. Functional bionanocomposite fibers of chitosan filled with cellulose nanofibers obtained by gel spinning. Polymers 2021, 13, 1563. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Abushammala, H.; Pontes, J.F.; Gomes, G.H.M.; Osorio-Madrazo, A.; Thiré, R.M.; Pereira, F.V.; Laborie, M.-P.G. Swelling, viscoelastic, and anatomical studies on ionic liquid-swollen Norway spruce as a screening tool toward ionosolv pulping. Holzforschung 2015, 69, 1059–1067. [Google Scholar] [CrossRef]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Novel preparation route for cellulose nanowhiskers. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2013. [Google Scholar]
- Kovacs, T.; Naish, V.; O’Connor, B.; Blaise, C.; Gagné, F.; Hall, L.; Trudeau, V.; Martel, P. An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 2010, 4, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, M.; Honkalampi, U.; Von Wright, A.; Sneck, A.; Hentze, H.P.; Sievänen, J.; Hiltunen, J.; Hellen, E. Nanofibrillar cellulose: In vitro study of cytotoxic and genotoxic properties. In Proceedings of the International Conferene on Nanotechnology for the Forest Products Industry, Espoo, Finland, 27–29 September 2010. [Google Scholar]
- Pértile, R.A.; Moreira, S.; Gil Da Costa, R.M.; Correia, A.; Guãrdao, L.; Gärtner, M.D.F.; Vilanova, M.; Gama, M. Bacterial cellulose: Long-Term biocompatibility studies. J. Biomater. Sci. Polym. Ed. 2012, 23, 1339–1354. [Google Scholar] [CrossRef] [Green Version]
- Kolakovic, R.; Laaksonen, T.; Peltonen, L.; Laukkanen, A.; Hirvonen, J. Spray-dried nanofibrillar cellulose microparticles for sustained drug release. Int. J. Pharm. 2012, 430, 47–55. [Google Scholar] [CrossRef]
- Eyholzer, C.; De Couraça, A.B.; Duc, F.; Bourban, P.E.; Tingaut, P.; Zimmermann, T.; Månson, J.A.E.; Oksman, K. Biocomposite hydrogels with carboxymethylated, nanofibrillated cellulose powder for replacement of the nucleus pulposus. Biomacromolecules 2011, 12, 1419–1427. [Google Scholar] [CrossRef]
- Borges, A.C.; Eyholzer, C.; Duc, F.; Bourban, P.-E.; Tingaut, P.; Zimmermann, T.; Pioletti, D.P.; Månson, J.-A.E. Nanofibrillated cellulose composite hydrogel for the replacement of the nucleus pulposus. Acta Biomater. 2011, 7, 3412–3421. [Google Scholar] [CrossRef] [Green Version]
- Tamo, A.K.; Doench, I.; Helguera, A.M.; Hoenders, D.; Walther, A.; Osorio Madrazo, A. Biodegradation of crystalline cellulose nanofibers by means of enzyme immobilized-alginate beads and microparticles. Polymers 2020, 12, 1522. [Google Scholar] [CrossRef]
- Arola, S.; Tammelin, T.; Setälä, H.; Tullila, A.; Linder, M.B. Immobilization–stabilization of proteins on nanofibrillated cellulose derivatives and their bioactive film formation. Biomacromolecules 2012, 13, 594–603. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Montembault, A.; Viguier, E.; Cachon, T. Hydrogel composites comprising chitosan and cellulose nanofibers. US Patent Application 16/980383, 18 February 2021. [Google Scholar]
- Osorio-Madrazo, A.; Fratzl, P.; David, L.; Urban, G.; Montembault, A.; Crepet, A.; Gorzelanny, C.; Mochales-Palau, C.; Heux, L.; Putaux, J.-L.; et al. Synthese und Charakterisierung von Biomaterialien (Polymere, Metalle, Keramiken, Komposite). P85: Hydrogel nanocomposite biomaterials for intervertebral disc tissue engineering. Preparation, characterization and application. Bionanomaterials 2015, 16, 236–255. [Google Scholar] [CrossRef]
- Omlor, G.W.; Nerlich, A.G.; Lorenz, H.; Bruckner, T.; Richter, W.; Pfeiffer, M.; Gühring, T. Injection of a polymerized hyaluronic acid/collagen hydrogel matrix in an in vivo porcine disc degeneration model. Eur. Spine J. 2012, 21, 1700–1708. [Google Scholar] [CrossRef] [Green Version]
- Endres, M.; Abbushi, A.; Thomale, U.W.; Cabraja, M.; Kroppenstedt, S.N.; Morawietz, L.; Casalis, P.A.; Zenclussen, M.L.; Lemke, A.-J.; Horn, P.; et al. Intervertebral disc regeneration after implantation of a cell-free bioresorbable implant in a rabbit disc degeneration model. Biomaterials 2010, 31, 5836–5841. [Google Scholar] [CrossRef]
- Hegewald, A.A.; Knecht, S.; Baumgartner, D.; Gerber, H.; Endres, M.; Kaps, C.; Stüssi, E.; Thomé, C. Biomechanical testing of a polymer-based biomaterial for the restoration of spinal stability after nucleotomy. J. Orthop. Surg. Res. 2009, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, S.; Matsuyama, Y.; Takahashi, K.; Satoh, T.; Koie, H.; Kanayama, K.; Tsuji, T.; Maruyama, K.; Imagama, S.; Sakai, Y.; et al. Regeneration of intervertebral disc by the intradiscal application of cross-linked hyaluronate hydrogel and cross-linked chondroitin sulfate hydrogel in a rabbit model of intervertebral disc injury. Bio. Med. Mater. Eng. 2009, 19, 421–429. [Google Scholar] [CrossRef]
- Rennerfeldt, D.A.; Renth, A.N.; Talata, Z.; Gehrke, S.H.; Detamore, M.S. Tuning mechanical performance of poly(ethylene glycol) and agarose interpenetrating network hydrogels for cartilage tissue engineering. Biomaterials 2013, 34, 8241–8257. [Google Scholar] [CrossRef] [Green Version]
- Chou, A.I.; Nicoll, S.B. Characterization of photocrosslinked alginate hydrogels for nucleus pulposus cell encapsulation. J. Biomed. Mater. Res. Part A 2009, 91, 187–194. [Google Scholar] [CrossRef]
- Varma, D.; Lin, H.; Long, R.; Gold, G.; Hecht, A.; Iatridis, J.; Nicoll, S. Thermoresponsive, redox-polymerized cellulosic hydrogels undergo in situ gelation and restore intervertebral disc biomechanics post discectomy. Eur. Cells Mater. 2018, 35, 300–317. [Google Scholar] [CrossRef]
- Sharifi, S.; Bulstra, S.K.; Grijpma, D.W.; Kuijer, R. Treatment of the degenerated intervertebral disc; closure, repair and regeneration of the annulus fibrosus. J. Tissue Eng. Regen. Med. 2014, 9, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Lall, A.; Tamo, A.K.; Doench, I.; David, L.; De Oliveira, P.N.; Gorzelanny, C.; Osorio-Madrazo, A. Nanoparticles and colloidal hydrogels of chitosan–caseinate polyelectrolyte complexes for drug-controlled release applications. Int. J. Mol. Sci. 2020, 21, 5602. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Covas, C.A.P.; Domard, A. Kinetics study of the solid-state acid hydrolysis of chitosan: Evolution of the crystallinity and macromolecular structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Lamarque, G.; Viton, C.; Domard, A. Comparative study of the first heterogeneous deacetylation of α- and β-chitins in a multistep process. Biomacromolecules 2004, 5, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.E.; Cardona, A.; Seung, H.S. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Graessley, W.W. The Entanglement Concept in Polymer Rheology; Springer Science and Business Media LLC: Berlin, Germany, 2006; pp. 1–179. [Google Scholar]
- Hwang, J.K.; Shin, H.H. Rheological properties of chitosan solutions. Korea Aust. Rheol. J. 2000, 12, 175–179. [Google Scholar]
- Cross, M.M. Rheology of non-Newtonian fluids: A new flow equation for pseudoplastic systems. J. Colloid Sci. 1965, 20, 417–437. [Google Scholar] [CrossRef]
- Calero, N.; Muñoz, J.; Ramírez, P.; Guerrero, A. Flow behaviour, linear viscoelasticity and surface properties of chitosan aqueous solutions. Food Hydrocoll. 2010, 24, 659–666. [Google Scholar] [CrossRef]
- Halimi, C.; Montembault, A.; Guerry, A.; Delair, T.; Viguier, E.; Fulchiron, R.; David, L. Chitosan solutions as injectable systems for dermal filler applications: Rheological characterization and biological evidence. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 2596–2599. [Google Scholar]
- Montembault, A.; Viton, C.; Domard, A. Rheometric study of the gelation of chitosan in a hydroalcoholic medium. Biomaterials 2005, 26, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Montembault, A.; Viton, A.C.; Domard, A. Rheometric study of the gelation of chitosan in aqueous solution without cross-linking agent. Biomacromolecules 2005, 6, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Kienzle-Sterzer, C.; Rodríguez-Sánchez, D.; Rha, C. Flow behavior of a cationic biopolymer: Chitosan. Polym. Bull. 1985, 13, 1–6. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [Green Version]
- Gharehkhani, S.; Sadeghinezhad, E.; Kazi, S.N.; Yarmand, H.; Badarudin, A.; Safaei, M.R.; Zubir, M.N.M. Basic effects of pulp refining on fiber properties—A review. Carbohydr. Polym. 2015, 115, 785–803. [Google Scholar] [CrossRef]
- Tanaka, R.; Saito, T.; Hondo, H.; Isogai, A. Influence of flexibility and dimensions of nanocelluloses on the flow properties of their aqueous dispersions. Biomacromolecules 2015, 16, 2127–2131. [Google Scholar] [CrossRef]
- Falcoz-Vigne, L.; Ogawa, Y.; Molina-Boisseau, S.; Nishiyama, Y.; Meyer, V.; Petit-Conil, M.; Mazeau, K.; Heux, L. Quantification of a tightly adsorbed monolayer of xylan on cellulose surface. Cellulose 2017, 24, 3725–3739. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Kurki-Suonio, S.; Schacher, F.H.; Hietala, S.; Rojas, O.; Ikkala, O. Water-resistant, transparent hybrid nanopaper by physical cross-linking with chitosan. Biomacromolecules 2015, 16, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Haider, S.; Al-Zeghayer, Y.; Ali, F.A.A.; Haider, A.; Mahmood, A.; Al-Masry, W.A.; Imran, M.; Aijaz, M.O. Highly aligned narrow diameter chitosan electrospun nanofibers. J. Polym. Res. 2013, 20, 1–11. [Google Scholar] [CrossRef]
- Robitzer, M.; David, L.; Rochas, C.; Di Renzo, F.; Quignard, F. Supercritically-dried alginate aerogels retain the fibrillar structure of the hydrogels. Macromol. Symp. 2008, 273, 80–84. [Google Scholar] [CrossRef]
- Sereni, N.; Enache, A.; Sudre, G.; Montembault, A.; Rochas, C.; Durand, P.; Perrard, M.-H.; Bozga, G.; Puaux, J.-P.; Delair, T.; et al. Dynamic structuration of physical chitosan hydrogels. Langmuir 2017, 33, 12697–12707. [Google Scholar] [CrossRef] [PubMed]
- Robitzer, M.; David, L.; Rochas, C.; Di Renzo, F.; Quignard, F. Nanostructure of calcium alginate aerogels obtained from multistep solvent exchange route. Langmuir 2008, 24, 12547–12552. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Sun, S.; Liu, Y.; Zhu, C.; Li, J.; Sun, J.; Wang, S.; Zhang, H. Characterization of crystal microstructure based on small angle X-ray scattering (SAXS) Technique. Molecules 2020, 25, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, S.M.; Kolsky, H. Stress birefringence in polyethylene. Proc. Phys. Soc. Sect. B 1951, 64, 119–125. [Google Scholar] [CrossRef]
- Boote, C.; Sturrock, E.J.; Attenburrow, G.E.; Meek, K.M. Psuedo-affine behaviour of collagen fibres during the uniaxial deformation of leather. J. Mater. Sci. 2002, 37, 3651–3656. [Google Scholar] [CrossRef]












| Formulation | CHI % (w/v) | CNFs % (w/w) | Needle Inner Diameter ID (μm) | Extrusion Pressure (bar) |
|---|---|---|---|---|
| F1 (CHI2) | 2 | 0 | 410 | 0.15 |
| F2 (CHI2/CNF0.4) | 2 | 0.4 | 410 | 0.25 |
| F3 (CHI3) | 3 | 0 | 410 | 0.35 |
| F4 (CHI3/CNF0.4) | 3 | 0.4 | 410 | 0.47 |
| F5 (CHI2) | 2 | 0 | 250 | 0.25 |
| F6 (CHI2/CNF0.4) | 2 | 0.4 | 250 | 0.35 |
| F7 (CHI3) | 3 | 0 | 250 | 0.70 |
| F8 (CHI3/CNF0.4) | 3 | 0.4 | 250 | 0.77 |
| Formulation | η0,CHI (Pa·s) | τCHI (s) | pCHI | s | η0,CNF (Pa·s) | τCNF (s) | pCNF |
|---|---|---|---|---|---|---|---|
| CHI2 | 83 | 1.0 | 0.73 | - | - | - | - |
| CHI2/CNF0.4 | 83 | 1.0 | 0.73 | 1.22 | 30.4 | 11.9 | 1.96 |
| CHI2/CNF0.5 | 83 | 1.0 | 0.73 | 1.56 | 87.8 | 9.6 | 1.46 |
| CHI2/CNF0.6 | 83 | 1.0 | 0.73 | 1.75 | 116.3 | 8.6 | 1.95 |
| CHI3 | 674 | 6.0 | 0.71 | - | - | - | - |
| CHI3/CNF0.4 | 674 | 6.0 | 0.71 | 1.11 | 39.3 | 8.7 | 6.55 |
| CHI3/CNF0.5 | 674 | 6.0 | 0.71 | 1.33 | 207.3 | 10.9 | 2.87 |
| CHI3/CNF0.6 | 674 | 6.0 | 0.71 | 1.88 | 752.1 | 13.9 | 1.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamdem Tamo, A.; Doench, I.; Walter, L.; Montembault, A.; Sudre, G.; David, L.; Morales-Helguera, A.; Selig, M.; Rolauffs, B.; Bernstein, A.; et al. Development of Bioinspired Functional Chitosan/Cellulose Nanofiber 3D Hydrogel Constructs by 3D Printing for Application in the Engineering of Mechanically Demanding Tissues. Polymers 2021, 13, 1663. https://doi.org/10.3390/polym13101663
Kamdem Tamo A, Doench I, Walter L, Montembault A, Sudre G, David L, Morales-Helguera A, Selig M, Rolauffs B, Bernstein A, et al. Development of Bioinspired Functional Chitosan/Cellulose Nanofiber 3D Hydrogel Constructs by 3D Printing for Application in the Engineering of Mechanically Demanding Tissues. Polymers. 2021; 13(10):1663. https://doi.org/10.3390/polym13101663
Chicago/Turabian StyleKamdem Tamo, Arnaud, Ingo Doench, Lukas Walter, Alexandra Montembault, Guillaume Sudre, Laurent David, Aliuska Morales-Helguera, Mischa Selig, Bernd Rolauffs, Anke Bernstein, and et al. 2021. "Development of Bioinspired Functional Chitosan/Cellulose Nanofiber 3D Hydrogel Constructs by 3D Printing for Application in the Engineering of Mechanically Demanding Tissues" Polymers 13, no. 10: 1663. https://doi.org/10.3390/polym13101663