Synthesis, Characterization, and Application of Carboxymethyl Cellulose from Asparagus Stalk End
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials Preparation
2.3. Extraction of Cellulose from Asparagus Stalk End
2.4. Carboxymethyl Cellulose (CMC) Synthesized from Asparagus Stalk End
2.5. Determination of the Degree of Substitution (DS) of CMCas
2.6. The Determination of Percentage of Residue on Ignition
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Viscosity of Cellulose and CMCas
2.9. Thermal Conductivity Measurements
2.10. X-ray Diffraction (XRD)
2.11. Scanning Electron Microscopy (SEM)
2.12. Color Characteristics
2.13. Preparation of CMCas Film
2.14. Mechanical Properties of CMCas Film
2.15. Statistical Analysis
3. Results and Discussion
3.1. Percent Yield of Carboxymethyl Cellulose from Asparagus Stalk End (CMCas)
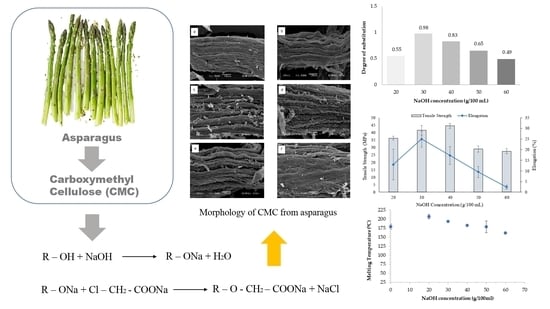
3.2. Degree of Substitution (DS) of CMCas
3.3. Fourier Transform Infrared Spectroscopy (FTIR) of Cellulose from Asparagus Stalk End and CMCas
3.4. Effect of Various NaOH Concentrations on Viscosity of CMCas
3.5. Effect of Various NaOH Concentrations on Thermal Properties of CMCas
3.6. X-ray Diffraction (XRD) of Cellulose from Asparagus Stalk End and CMCas
3.7. Morphology of Cellulose from Asparagus Stalk End and CMCas
3.8. Color of Cellulose from Asparagus Stalk End and CMCas
3.9. Mechanical Properties of CMCas Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nindo, C.; Sun, T.; Wang, S.; Tang, J.; Powers, J. Evaluation of drying technologies for retention of physical quality and antioxidants in asparagus (Asparagus officinalis, L.). LWT Food Sci. Technol. 2003, 36, 507–516. [Google Scholar] [CrossRef]
- Huang, Y.; Kennedy, J.; Peng., Y.; Wang, X.; Yuan, X.; Zhao, B.; Zhao, Q. Optimization of ultrasonic circulating extraction of polysaccharides from Asparagus officinalis using response surface methodology. Int. J. Biol. Macromol. 2011, 49, 181–187. [Google Scholar]
- Guo, Q.; Wang, N.; Liu, H.; Li, Z.; Lu, L.; Wang, C. The bioactive compounds and biological functions of Asparagus officinalis L.—A review. J. Funct. Foods 2020, 65, 103727. [Google Scholar] [CrossRef]
- Noperi-Mosqueda, L.C.; López-Moreno, F.J.; Navarro-León, E.; Sánchez, E.; Blasco, B.; Moreno, D.A.; Soriano, T.; Ruiz, J.M. Effects of asparagus decline on nutrients and phenolic compounds, spear quality, and allelopathy. Sci. Hortic. 2020, 261, 109029. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Jaramillo-Carmona, S.; Rodríguez-Gutiérrez, G.; Rodríguez-Arcos, R.; Fernández-Bolaños, J.; Guillén-Bejarano, R.; Espejo-Calvo, J.A.; Jiménez-Araujo, A. Effect of the extraction method on phytochemical composition and antioxidant activity of high dietary fibre powders obtained from asparagus by-products. Food Chem. 2009, 116, 484–490. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing nanocellulose to bulk materials: A review. Cellulose 2019, 26, 7585–7617. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semimicro determination of cellulose in biological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl Cellulose Film from Durian Rind. LWT Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Grand View Research, Inc. Carboxymethyl Cellulose Market Analysis By Application (Cosmetics & Pharmaceuticals, Food & Beverages, Oil & Gas, Paper & Board, Detergents), By Region, And Segment Forecasts, 2018–2025 (Report No GVR-1-68038-463-5); Grand View Research, Inc.: San Francisco, CA, USA, 2017; Available online: https://www.grandviewresearch.com/industry-analysis/carboxymethyl-cellulose-cmc- (accessed on 8 November 2020).
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimisation of Reaction Condition for Preparing Carboxymethyl Cellulose from Sago Waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and Characterization of Carboxymethyl Cellulose Powder and Films from Mimosa Pigra Peel. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- Haleem, N.; Arshad, M.; Shahid, M.; Tahir, M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014, 113, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Mondal, I.H.; Yeasmin, M.S.; Rahman, S. Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int. J. Biol. Macromol. 2015, 79, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Liu, H.-J.; Zhang, G.-G. Preparation of Carboxymethyl Cellulose from Corncob. Procedia Environ. Sci. 2016, 31, 98–102. [Google Scholar] [CrossRef] [Green Version]
- Karataş, M.; Arslan, N. Flow behaviours of cellulose and carboxymethyl cellulose from grapefruit peel. Food Hydrocoll. 2016, 58, 235–245. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Rice stubble as a new biopolymer source to produce carboxymethyl cellulose-blended films. Carbohydr. Polym. 2017, 171, 94–101. [Google Scholar] [CrossRef]
- Rachtanapun, P. Blended Films of Carboxymethyl Cellulose from Papaya Peel/Corn Starch Film Blends. Kasetsart J. 2009, 43, 259–266. [Google Scholar]
- Kassab, Z.; Syafri, E.; Tamraoui, Y.; Hannache, H.; Qaiss, A.E.K.; Achaby, M.E. Characteristics of sulfated and carboxylated cellulose nanocrystals extracted from Juncus plant stems. Int. J. Biol. Macromol. 2020, 154, 1419–1425. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Kumthai, S.; Mulkarat, N.; Pintajam, N.; Suriyatem, R. Value added of mulberry paper waste by carboxymethylation for preparation a packaging film. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012081. [Google Scholar] [CrossRef] [Green Version]
- Inphonlek, S.; Sunintaboon, P.; Leonard, M.; Durand, A. Chitosan/carboxymethylcellulose-stabilized poly(lactide-co-glycolide) particles as bio-based drug delivery carriers. Carbohydr. Polym. 2020, 242, 116417. [Google Scholar] [CrossRef]
- Koneru, A.; Dharmalingam, K.; Anandalaksh, R. Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose–grapefruit seed extract nanoparticles for potential wound healing applications. Int. J. Biol. Macromol. 2020, 148, 833–842. [Google Scholar] [CrossRef]
- Calegari, F.; Da Silva, B.C.; Tedim, J.; Ferreira, M.; Berton, M.A.; Marino, C.E. Benzotriazole encapsulation in spray-dried carboxymethylcellulose microspheres for active corrosion protection of carbon steel. Prog. Org. Coatings 2020, 138, 105329. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Eitssayeam, S.; Pengpat, K. Study of Carboxymethyl Cellulose from Papaya Peels Binder in Ceramics. Adv. Mater. Res. 2010, 93–94, 17–21. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric acid cross linked β-cyclodextrin/carboxymethyl cellulose hydrogel films for controlled delivery of poorly soluble drugs. Carbohydr. Polym. 2017, 164, 339–348. [Google Scholar] [CrossRef]
- Gregorova, A.; Saha, N.; Kitano, T.; Saha, P. Hydrothermal effect and mechanical stress properties of carboxymethylcellulose based hydrogel food packaging. Carbohydr. Polym. 2015, 117, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Kordowska-Wiater, M.; Złotek, U.; Skrzypek, T. Antifungal resistance and physicochemical attributes of apricots coated with potassium sorbate added carboxymethyl cellulose-based emulsion. Int. J. Food Sci. Technol. 2018, 53, 728–734. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Kordowska-Wiater, M.; Kałwa, K.; Skrzypek, T.; Sikora, M.; Łupina, K. Physiological, qualitative, and microbiological changes of minimally processed Brussels sprouts in response to coating with carboxymethyl cellulose/candelilla wax emulsion. J. Food Process. Preserv. 2019, 43, e14004. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Wang, Q.; Tang, Y.; Li, F.; Zhao, J.; Zhang, Y.; Ming, J. Combined effect of carboxymethylcellulose and salt on structural properties of wheat gluten proteins. Food Hydrocoll. 2019, 97, 105189. [Google Scholar] [CrossRef]
- Yuliarti, O.; Mei, K.H.; Ting, Z.K.X.; Yi, K.Y. Influence of combination carboxymethylcellulose and pectin on the stability of acidified milk drinks. Food Hydrocoll. 2019, 89, 216–223. [Google Scholar] [CrossRef]
- Rhim, J.; Wu, Y.; Weller, C.; Schnepf, M. Physical character-istics of a composite film of soy protein isolate and propylene-glycol alginate. J. Food Sci. 1999, 64, 149–152. [Google Scholar] [CrossRef]
- ASTM (D 882-80a). Standards test methods for tensile properties of thin plastic sheeting. In Annual Book of ASTM Standard; ASTM: West Conshohochen, PA, USA, 1995; pp. 182–190. [Google Scholar]
- Adinugraha, M.P.; Marseno, D.W.; Hayadi. Synthesis and characterization of sodium carboxymethyl cellulose from cavendish banana pseudo stem (Musa cavendishii LAMBERT). Carbohydr. Polym. 2005, 62, 164–169. [Google Scholar] [CrossRef]
- Kirk, R.E.; Othmer, D.F. Cellulose in Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 1967; Volume 4, pp. 593–683. [Google Scholar]
- Célino, A.; Gonçalves, O.; Jacquemin, F.; Fréour, S. Qualitative and quantitative assessment of water sorption in natural fibres using ATR-FTIR spectroscopy. Carbohydr. Polym. 2014, 101, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suriyatem, R.; Noikang, N.; Kankam, T.; Jantanasakulwong, K.; Leksawasdi, N.; Phimolsiripol, Y.; Insomphun, C.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; et al. Physical Properties of Carboxymethyl Cellulose from Palm Bunch and Bagasse Agricultural Wastes: Effect of Delignification with Hydrogen Peroxide. Polymers 2020, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Asl, S.A.; Mousavi, M.; Labbaf, M. Synthesis and characterization of carboxymethyl cellulose from sugarcane bagasse. J. Food Process. Technol. 2017, 8, 687. [Google Scholar]
- El-sayed, S.; Mahmoud, K.H.; Fatah, A.A.; Hassen, A. DSC, TGA and dielectric properties of carboxymethyl cellulose/polyvinyl alcohol blends. Phys. B Condens. Matter 2011, 406, 4068–4076. [Google Scholar] [CrossRef]
- Teramoto, Y. Functional Thermoplastic Materials from Derivatives of Cellulose and Related Structural Polysaccharides. Molecules 2015, 20, 5487–5527. [Google Scholar] [CrossRef] [Green Version]
- Sangseethong, K.; Chatakanonda, P.; Wansuksri, R.; Sriroth, K. Influence of reaction parameters on carboxymethylation of rice starches with varying amylose contents. Carbohydr. Polym. 2015, 115, 186–192. [Google Scholar] [CrossRef]







| Type of Sample | L* | a* | b* | WI | YI | ∆E | Hab |
|---|---|---|---|---|---|---|---|
| Cellulose | 71.51 ± 0.37 b | 3.01 ± 0.05 c | 21.31 ± 0.26 b | 61.83 ± 0.33 b | 31.21 ± 1.89 c | 29.62 ± 0.24 ns | 5.87 ± 0.17 a |
| 20 g/100 mL NaOH-CMCas | 73.88 ± 0.45 a | 2.69 ± 0.28 d | 20.77 ± 0.50 c | 64.54 ± 0.55 a | 45.02 ± 1.05 a | 37.46 ± 21.89 | 6.33 ± 0.90 a |
| 30 g/100 mL NaOH-CMCas | 71.78 ±0.74 b | 2.87 ± 0.08 c,d | 21.70 ± 0.38 b | 61.99 ± 0.62 b | 45.02 ± 1.05 a | 29.67 ± 0.35 | 6.31 ± 0.10 a |
| 40 g/100 mL NaOH-CMCas | 68.51 ± 0.44 c,d | 3.69 ± 0.25 b | 22.78 ± 0.17 a | 58.52 ± 0.42 d | 45.20 ± 1.01 a | 32.90 ± 0.32 | 4.94 ± 0.14 c,d |
| 50 g/100 mL NaOH-CMCas | 69.30 ± 0.47 c | 3.54 ± 0.14 b | 21.56 ± 0.29 b | 59.79 ±0.35 c | 41.52 ± 1.66 b | 31.50 ± 0.44 | 5.06 ± 0.17 b |
| 60 g/100 mL NaOH-CMCas | 67.97 ± 1.33 d | 4.00 ± 0.16 a | 21.28 ± 0.31 b | 58.34 ± 1.25 d | 40.17 ± 1.59 b | 32.43 ± 0.94 | 4.42 ± 0.22 d |
| Type of Film | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| 20 g/100 mL NaOH-CMCas | 36.30 ± 1.32 c | 13.00 ± 7.22 c |
| 30 g/100 mL NaOH-CMCas | 41.78 ± 3.28 b | 24.99 ± 3.79 a |
| 40 g/100 mL NaOH-CMCas | 44.59 ± 1.73 a | 17.32 ± 4.21 b |
| 50 g/100 mL NaOH-CMCas | 28.97 ± 2.06 d | 9.52 ± 2.57 c |
| 60 g/100 mL NaOH-CMCas | 27.55 ± 1.72 d | 2.38 ± 0.88 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, Characterization, and Application of Carboxymethyl Cellulose from Asparagus Stalk End. Polymers 2021, 13, 81. https://doi.org/10.3390/polym13010081
Klunklin W, Jantanasakulwong K, Phimolsiripol Y, Leksawasdi N, Seesuriyachan P, Chaiyaso T, Insomphun C, Phongthai S, Jantrawut P, Sommano SR, et al. Synthesis, Characterization, and Application of Carboxymethyl Cellulose from Asparagus Stalk End. Polymers. 2021; 13(1):81. https://doi.org/10.3390/polym13010081
Chicago/Turabian StyleKlunklin, Warinporn, Kittisak Jantanasakulwong, Yuthana Phimolsiripol, Noppol Leksawasdi, Phisit Seesuriyachan, Thanongsak Chaiyaso, Chayatip Insomphun, Suphat Phongthai, Pensak Jantrawut, Sarana Rose Sommano, and et al. 2021. "Synthesis, Characterization, and Application of Carboxymethyl Cellulose from Asparagus Stalk End" Polymers 13, no. 1: 81. https://doi.org/10.3390/polym13010081