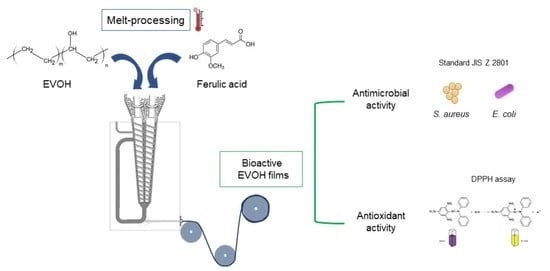
Melt-Processed Bioactive EVOH Films Incorporated with Ferulic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Stability of Trans-Ferulic Acid
2.3. Film Formation
2.4. Quantification of Ferulic Acid in EVOH-Based Films after Processing
2.5. Structural and Morphological Properties of EVOH Blended with FA
2.5.1. ATR-FTIR Analysis
2.5.2. Morphological Analysis
2.6. Thermal Characterization
2.6.1. Differential Scanning Calorimetry (DSC)
2.6.2. Thermogravimetric Analysis (TGA)
2.7. Functional Properties of the Films
2.7.1. Thickness and Optical Properties
2.7.2. Water Contact Angle
2.7.3. Barrier Properties
2.7.4. Mechanical Properties
2.8. Antioxidant Properties
2.8.1. Antioxidant Activity of Films
2.8.2. Antioxidant Activity of Ferulic Acid Released into Food Simulants
2.9. Antimicrobial Activity
2.10. Statistical Analysis
3. Results
3.1. Thermal Stability of Trans-Ferulic Acid
3.2. Quantification of Ferulic Acid in EVOH-Based Films
3.3. Structural and Morphological Properties of the Films
3.3.1. FTIR Characterization
3.3.2. SEM Studies
3.4. Thermal Analysis
3.4.1. Differential Scanning Calorimetry
3.4.2. Thermogravimetric Analysis
3.5. Functional Properties of Films
3.5.1. Thickness and Optical Properties
3.5.2. Surface Wettability
3.5.3. Mechanical and Barrier Properties
3.6. Antioxidant Activity
3.7. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Gong, Y.; Parker, R.S.; Richards, M.P. Factors affecting lipid oxidation in breast and thigh muscle from chicken, turkey and duck. J. Food Biochem. 2010, 34, 869–885. [Google Scholar] [CrossRef]
- Delgado-Adámez, J.; Bote, E.; Parra-Testal, V.; Martín, M.J.; Ramírez, R. Effect of the olive leaf extracts in vitro and in active packaging of sliced Iberian pork loin. Packag. Technol. Sci. 2016, 29, 649–660. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Verma, A.K.; Siddiqui, M.W. Natural Antioxidants: Applications in Foods of Animal Origin; Apple Academic Press: Palm Bay, FL, USA, 2017; ISBN 9781771884600. [Google Scholar]
- Luzi, F.; Puglia, D.; Dominici, F.; Fortunati, E.; Giovanale, G.; Balestra, G.M.; Torre, L. Effect of gallic acid and umbelliferone on thermal, mechanical, antioxidant and antimicrobial properties of poly (vinyl alcohol-co-ethylene) films. Polym. Degrad. Stab. 2018, 152, 162–176. [Google Scholar] [CrossRef]
- Pateiro, M.; Domínguez, R.; Bermúdez, R.; Munekata, P.E.S.; Zhang, W.; Gagaoua, M.; Lorenzo, J.M. Antioxidant active packaging systems to extend the shelf life of sliced cooked ham. Curr. Res. Food Sci. 2019, 1, 24–30. [Google Scholar] [CrossRef]
- Rodríguez, G.M.; Sibaja, J.C.; Espitia, P.J.P.; Otoni, C.G. Antioxidant active packaging based on papaya edible films incorporated with Moringa oleifera and ascorbic acid for food preservation. Food Hydrocoll. 2019, 103, 105630. [Google Scholar] [CrossRef]
- Peponi, L.; Arrieta, M.P.; Mujica-Garcia, A.; López, D. Smart Polymers. In Modification of Polymer Properties; William Andrew Publishing: New York, NY, USA, 2017; ISBN 9780323443982. [Google Scholar]
- Arrieta, M.P.; Sessini, V.; Peponi, L. Biodegradable poly(ester-urethane) incorporated with catechin with shape memory and antioxidant activity for food packaging. Eur. Polym. J. 2017, 94, 111–124. [Google Scholar] [CrossRef]
- Gavara, R.; Catalá, R.; López Carballo, G.; Cerisuelo, J.P.; Dominguez, I.; Muriel-Galet, V.; Hernandez-Muñoz, P. Use of EVOH for Food Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-08-100596-5. [Google Scholar]
- Lagarón, J.M.; Giménez, E.; Saura, J.J.; Gavara, R. Phase morphology, crystallinity and mechanical properties of binary blends of high barrier ethylene–vinyl alcohol copolymer and amorphous polyamide and a polyamide-containing ionomer. Polymer (Guildf) 2001, 42, 7381–7394. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M.; Giménez, E.; Cava, D.; Hernandez-Muñoz, P.; Yamamoto, T.; Gavara, R. Morphological alterations induced by temperature and humidity in ethylene−vinyl alcohol copolymers. Macromolecules 2003, 36, 9467–9476. [Google Scholar] [CrossRef]
- Nam, s.; Scanlon, M.G.; Han, J.; Izydorczyk, M. Extrusion of pea starch containing lysozyme and determination of antimicrobial activity. J. Food Sci. 2007, 72, E477–E484. [Google Scholar] [CrossRef] [PubMed]
- Nobile, M.A.; Conte, A.; Buonocore, G.G.; Anna Lucia, I.; Massaro, A.; Panza, O. Active packaging by extrusion processing of recyclable and biodegradable polymer. J. Food Eng. 2009, 93, 1–6. [Google Scholar] [CrossRef]
- Wang, Q.J.; Gao, X.; Gong, H.; Lin, X.R.; Saint-Leger, D.; Senee, J. Chemical stability and degradation mechanisms of ferulic acid (F.A) within various cosmetic formulations. J. Cosmet. Sci. 2011, 62, 483–503. [Google Scholar] [PubMed]
- Kim, J.K.; Park, S.U. A recent overview on the biological and pharmacological activities of ferulic acid. EXCLI J. 2019, 18, 132–138. [Google Scholar] [PubMed]
- López-de-Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem. 2012, 131, 1376–1384. [Google Scholar] [CrossRef]
- GilakHakimabadi, S.; Ehsani, M.; Khonakdar, H.A.; Ghaffari, M.; Jafari, S.H. Controlled release of ferulic acid from active packaging based on LDPE/EVA blend: Experimental and modeling. Food Packag. Shelf Life 2019, 22, 100392. [Google Scholar] [CrossRef]
- Faisant, J.B.; Aït-Kadi, A.; Bousmina, M.; Descheˆnes, L. Morphology, thermomechanical and barrier properties of polypropylene-ethylene vinyl alcohol blends. Polymer (Guildf) 1998, 39, 533–545. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. ASTM D 2244: Standard Practice for Calculation of Color Tolerances and Color Differences from; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM International. Standard Practice for Calculating Yellowness and Whiteness Indices from Instrumentally Measured Color Coordinates; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM International. Standard test method for water vapor transmission rate through plastic film and sheeting using a modulated infrared sensor. Astm 2015. [Google Scholar] [CrossRef]
- ASTM International. ASTM D3985-17: Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ISO. ISO 527:2012 Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Okada, Y.; Okada, M. Scavenging effect of water-soluble proteins in broad beans on free radicals and active oxygen species. J. Agric. Food Chem. 1998, 46, 401–406. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Cejudo-Bastante, C.; Cran, M.J.; Heredia, F.J.; Bigger, S.W. Optical, structural, mechanical and thermal characterization of antioxidant ethylene vinyl alcohol copolymer films containing betalain-rich beetroot. Food Packag. Shelf Life 2020, 24, 100502. [Google Scholar] [CrossRef]
- Ramos, M.; Beltran, A.; Fortunati, E.; Peltzer, M.A.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled release of thymol from poly(Lactic acid)-based silver nanocomposite films with antibacterial and antioxidant activity. Antioxidants 2020, 9, 395. [Google Scholar] [CrossRef]
- Japanese Standards Association. JIS Z 2801 2000—Antimicrobial Products—Test for Antimicrobial Activity and Efficacy; Japanese Standards Association: Tokyo, Japan, 2000. [Google Scholar]
- Fiddler, W.; Parker, W.E.; Wasserman, A.E.; Doerr, R.C. Thermal decomposition of ferulic acid. J. Agric. Food Chem. 1967, 15, 757–761. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Development of novel nano-biocomposite antioxidant films based on poly (lactic acid) and thymol for active packaging. Food Chem. 2014, 162, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, W.; Potiszil, C.; Watson, J.S.; Sephton, M.A. Sporopollenin, a natural copolymer, is robust under high hydrostatic pressure. Macromol. Chem. Phys. 2016, 217, 2494–2500. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, S.; Sundaraganesan, N.; Manoharan, S. Molecular structure, spectroscopic studies and first-order molecular hyperpolarizabilities of ferulic acid by density functional study. Spectrochim. Acta Part A 2009, 74, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chaves, M.; Ruiz, C.; Cerrada, M.L.; Fernández-García, M. Novel glycopolymers containing aminosaccharide pendant groups by chemical modification of ethylene-vinyl alcohol copolymers. Polymer (Guildf) 2008, 49, 2801–2807. [Google Scholar] [CrossRef]
- Xu, D.; Lu, J.; Yan, S.; Xiao, R. Aminated EVOH nanofiber membranes for Cr(vi) adsorption from aqueous solution. RSC Adv. 2018, 8, 742–751. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Lei, H.; Xie, C.S. The effect of nucleating agent on the crystalline morphology of polypropylene (PP). Mater. Des. 2003, 24, 227–230. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Giménez, E.; Saura, J.J. Degradation of high barrier ethylene-vinyl alcohol copolymer under mild thermal-oxidative conditions studied by thermal analysis and infrared spectroscopy. Polym. Int. 2001, 50, 635–642. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peponi, L.; López, D.; Fernández-García, M. Recovery of yerba mate (Ilex paraguariensis) residue for the development of PLA-based bionanocomposite films. Ind. Crops Prod. 2018, 111, 317–328. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Han, S.H.; Kim, Y.S. Invetigation of hydrophobic properties of PSII-modified EVOH, LLDPE, and PET Films. J. Surf. Anal. 2005, 12, 258–262. [Google Scholar]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent updates on the barrier properties of Ethylene Vinyl Alcohol Copolymer (EVOH): A review. Polym. Rev. 2018, 58. [Google Scholar] [CrossRef] [Green Version]
- Lagarón, J.M.; Giménez, E.; Altava, B.; Del-Valle, V.; Gavara, R. Characterization of extruded ethylene-vinyl alcohol copolymer based barrier blends with interest in food packaging applications. Macromol. Symp. 2003, 198, 473–482. [Google Scholar] [CrossRef]
- Gnanasekharan, V.; Floros, J.D. Migration and sorption phenomena in packaged foods. Crit. Rev. Food Sci. Nutr. 1997, 37, 519–559. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- López De Dicastillo, C.; Nerín, C.; Alfaro, P.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Development of new antioxidant active packaging films based on ethylene vinyl alcohol copolymer (EVOH) and green tea extract. J. Agric. Food Chem. 2011, 59, 7832–7840. [Google Scholar] [CrossRef]
- Luzi, F.; Pannucci, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R.; Puglia, D. Gallic acid and quercetin as intelligent and active ingredients in poly(vinyl alcohol) films for food packaging. Polymers 2019, 11, 1999. [Google Scholar] [CrossRef] [Green Version]
- Valdés García, A.; Juárez Serrano, N.; Beltrán Sanahuja, A.; Garrigós, M.C. Novel antioxidant packaging films based on poly(ε-caprolactone) and almond skin extract: Development and effect on the oxidative stability of fried almonds. Antioxidants 2020, 9, 629. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial activity of gallic acid against food-related Pseudomonas strains and its use as biocontrol tool to improve the shelf life of fresh black truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef] [PubMed]






| Formulation | Code | Ferulic Acid (FA) (wt.%) |
|---|---|---|
| Ethylene vinyl alcohol (EVOH) | EVOH | Not detected |
| EVOH + FA 0.25 wt.% | EVOH-FA0.25 | 0.11 ± 0.01 |
| EVOH + FA 0.5 wt.% | EVOH-FA0.5 | 0.37 ± 0.02 |
| EVOH + FA 0.75 wt.% | EVOH-FA0.75 | 0.52 ± 0.05 |
| EVOH + FA 1 wt.% | EVOH-FA1 | 0.71 ± 0.04 |
| Cooling | Second Heating | ||||||
|---|---|---|---|---|---|---|---|
| Formulation | Tg (°C) | Tc (°C) | ∆Hc (J/g) | Tg (°C) | Tm (°C) | ∆Hm (J/g) | χc (%) |
| EVOH | 50 | 145 | 67 | 54 | 165 | 70 | 31 |
| EVOH-FA0.25 | 49 | 144 | 68 | 51 | 164 | 72 | 31 |
| EVOH-FA0.5 | 47 | 143 | 63 | 50 | 163 | 64 | 29 |
| EVOH-FA0.75 | 46 | 143 | 65 | 48 | 163 | 70 | 30 |
| EVOH-FA1 | 45 | 143 | 65 | 48 | 163 | 69 | 30 |
| Formulation | T5% (°C) | TmaxI (°C) | TmaxII (°C) |
|---|---|---|---|
| EVOH | 348 | 397 | 457 |
| EVOH-FA0.25 | 349 | 405 | 457 |
| EVOH-FA0.5 | 349 | 408 | 458 |
| EVOH-FA0.75 | 352 | 415 | 460 |
| EVOH-FA1 | 351 | 413 | 460 |
| Formulation | Thickness (µm) | L* (D65) | Cab* | h°ab | ΔE | YI (E313) | UV (190–399 nm) | Visible (400–800 nm) |
|---|---|---|---|---|---|---|---|---|
| EVOH | 83 ± 3 | 89.6 ± 0.2 | 0.2 ± 0.0 | 31.1 ± 0.8 | - | -0.9 ± 0.1 | 181 | 64 |
| EVOH-FA0.25 | 78 ± 5 | 89.5 ± 0.3 | 2.3 ± 0.2 | 96.3 ± 0.8 | 2.2 ± 0.1 | 2.7 ± 0.2 | 1036 | 61 |
| EVOH-FA0.5 | 81 ± 4 | 90.3 ± 0.2 | 2.7 ± 0.2 | 98.7 ± 0.4 | 2.7 ± 0.2 | 3.3 ± 0.4 | 1066 | 63 |
| EVOH-FA0.75 | 84 ± 7 | 89.8 ± 0.3 | 3.6 ± 0.2 | 96.4 ± 0.3 | 3.5 ± 0.2 | 4.7 ± 0.3 | 1062 | 68 |
| EVOH-FA1 | 77 ± 3 | 90.2 ± 0.3 | 2.9 ± 0.3 | 95.1 ± 1.6 | 2.9 ± 0.2 | 3.7 ± 0.4 | 1113 | 82 |
| Formulation | E (GPa) | TS (MPa) | εB (%) | OTR (mL/m2 Day) | WVTR (g/m2 Day) | Θ (°) |
|---|---|---|---|---|---|---|
| EVOH | 3.2 ± 0.0 a,b | 62 ± 1 a | 33 ± 3 a | 1.55 ± 0.26 | 0.20 ± 0.05 | 51 ± 4 a |
| EVOH-FA0.25 | 3.2 ± 0.0 a | 61 ± 3 a | 38 ± 6 a,b | 1.16 ± 0.17 | 0.16 ± 0.02 | 71 ± 4 b |
| EVOH-FA0.5 | 3.3 ± 0.1 b | 63 ± 2 a | 41 ± 2 a,b | 1.12 ± 0.11 | 0.23 ± 0.03 | 64 ± 2 b,c |
| EVOH-FA0.75 | 3.3 ± 0.0 a,b | 60 ± 1 a | 43 ± 7 b | 1.91 ± 0.38 | 0.28 ± 0.04 | 62 ± 5 c |
| EVOH-FA1 | 3.3 ± 0.0 b | 64 ± 2 a | 54 ± 4 c | 2.09 ± 0.56 | 0.32 ± 0.09 | 61 ± 6 c |
| JIS Z 2801 | E. coli | S. aureus | ||
|---|---|---|---|---|
| Formulation | Log CFU/cm2 Mean | Antibacterial Activity (R) | Log CFU/cm2 Mean | Antibacterial Activity (R) |
| EVOH | 6.66 ± 0.12 | - | 5.16 ± 0.00 | - |
| EVOH-FA0.25 | 4.99 ± 0.69 | 1.6 | 5.22 ± 0.12 | - |
| EVOH-FA0.5 | 4.01 ± 0.11 | 2.6 | 4.49 ± 0.24 | 0.7 |
| EVOH-FA0.75 | 3.47 ± 0.11 | 3.2 | 4.34 ± 0.22 | 0.8 |
| EVOH-FA1 | 2.97 ± 0.08 | 3.7 | 4.03 ± 0.22 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragón-Gutiérrez, A.; Rosa, E.; Gallur, M.; López, D.; Hernández-Muñoz, P.; Gavara, R. Melt-Processed Bioactive EVOH Films Incorporated with Ferulic Acid. Polymers 2021, 13, 68. https://doi.org/10.3390/polym13010068
Aragón-Gutiérrez A, Rosa E, Gallur M, López D, Hernández-Muñoz P, Gavara R. Melt-Processed Bioactive EVOH Films Incorporated with Ferulic Acid. Polymers. 2021; 13(1):68. https://doi.org/10.3390/polym13010068
Chicago/Turabian StyleAragón-Gutiérrez, Alejandro, Estela Rosa, Miriam Gallur, Daniel López, Pilar Hernández-Muñoz, and Rafael Gavara. 2021. "Melt-Processed Bioactive EVOH Films Incorporated with Ferulic Acid" Polymers 13, no. 1: 68. https://doi.org/10.3390/polym13010068