1. Introduction
Temperature-modulated scanning calorimetry is a method that applies a periodic modulation in temperature to the heating or cooling temperature scan at a constant rate or isothermal holding [
1]; typical modes of modulation are shown in
Figure 1. The response appearing in the heat flow is comprised of the change in the amplitude and the time lag. A dynamic heat capacity of complex quantity
can be determined from the response with the magnitude
and the phase angle
determined from the amplitude and the time lag. The method has been mainly employed in glass transition to examine the relaxation phenomena as temperature and frequency dispersions [
2]. Because temperature modulation also influences the degree of supercooling or superheating of the kinetics of first-order phase transitions, we can obtain valuable information regarding the transition kinetics [
3,
4,
5,
6,
7,
8,
9,
10] such as crystallization [
3,
9], melting [
4,
6,
9], and solid-solid phase transitions [
7,
10] of polymers [
3,
4,
6,
7,
8], water [
9], and metal alloy [
10]; the heating only condition of
Figure 1 is utilized to examine the one-way irreversible transition on heating. The typical responses are schematically shown in
Figure 2 for the case of crystalline polymer, which is prepared by quenching and in the state of glass before the heating run.
In
Figure 2, an effective dynamic heat capacity is shown with the contribution of the processes of glass transition, cold crystallization, and melting, which are identified in
Figure 2a by the respective changes in
with the mean heat flow HF divided by the mean underlying heating rate
and converted to an apparent heat capacity.
- (1)
glass transition: a step wise change in both of and and a peak in . The dependences on applied period of modulation characterize the relaxation phenomena of the glass transition.
- (2)
cold crystallization on heating from the glass: large exothermic peak in , very small change in , and a negative peak in .
- (3)
melting region: large peaks in , , and .
Those responses of the kinetics of first-order phase transitions of crystallization and melting are characterized by the time constants
of the kinetics, which determine their dependences on applied modulation period
, approximated in the following manner [
4,
5,
6],
where
represents the sample heat capacity,
the heat flow rate of transition with negative sign for endothermic process,
imaginary unit,
the angular frequency of modulation, and
the time constant depending on the underlying heating rate
.
Firstly, very small change in
on cold crystallization suggests that the time constant of crystallization kinetics is much longer than the applied modulation period; it is reasonable because the completion of crystallization usually needs much longer time than the applied modulation period. Then, the dependence of
on the period
becomes monotonically increasing as expected from Equation (1). On the other hand, the negative peak in
suggests that the cold crystallization is a process under deep non-equilibrium state and this exothermic process becomes faster on heating [
4].
Secondly, the strong dependence on the applied modulation period of the broad and large peaks of
and
on melting suggests that the time constant of the melting kinetics is in the range of the applied modulation periods. Actually, the dependence of
on applied modulation period becomes non-monotonic. On the other hand, as a limiting behavior for
(
),
approaches to the sum of true heat capacity
and the heat flow rate of transition divided by
, namely
, as shown in Equation (1) [
4]. Therefore,
is monotonically increasing with longer
. However, as the consequence of a complex behavior of metastable chain-folded polymer crystals,
in the melting region can become larger than
, as shown in
Figure 2 [
4]. This behavior is caused by an exothermic process of recrystallization that proceeds simultaneously with melting, namely,
With very small response of recrystallization appearing in
as the nature of crystallization, the melting kinetics solely determines the magnitude of
in the melting region, and hence
can become larger than
. It is noted that the difference
is named non-reversing heat flow and utilized for discriminating different types of kinetic response in general [
1]; this behavior of melting and recrystallization kinetics is one of the examples.
The method has been employed using conventional differential scanning calorimetry (DSC). As typically shown in
Figure 2, the following characteristics of the melting region of polymer crystals has been clarified experimentally [
4].
- (1)
Melting and recrystallization proceed in parallel.
- (2)
Those processes start at much lower temperature region than the melting peak of .
The recent development of fast-scan calorimetry (FSC) with a chip sensor [
11,
12] enables the examination of the method at fast-scan rates [
2,
13,
14,
15].
As another characteristic feature of complex melting of polymer crystals, double- or multiple-melting peak appears with a number of crystalline polymers [
16]. The origin of the peaks has been controversially discussed [
17,
18] in terms of the possibilities of distinctive true melting peaks as prepared crystals and the consequence of reorganization and/or melting-recrystallization-remelting. The identification of the true melting point of the original crystals is most important to characterize the thermal property of crystalline polymers [
16].
For crystalline polyesters, such as poly(ethylene terephthalate) (PET) [
18], poly(trimethylene terephthalate) [
19], and poly(butylene terephthalate) (PBT) [
20], by applying fast-scan calorimetry, it has been shown that the higher-melting peak shifts to lower temperature and the two peaks merge into a single peak at sufficiently high heating rates. This behavior indicated that the higher-temperature melting peak appeared as a result of melting-recrystallization-remelting, which became more pronounced at slower heating rates [
16]. Then, the lower-temperature melting peak represents the melting of the original crystals formed during the sample preparation.
As reviewed in the above, we can differentiate melting and recrystallization kinetics having different time constants by using a temperature modulation method. A recent study of temperature-modulated fast-scan calorimetry (T-M FSC) on the double melting peaks of PET [
15] supported the above conclusion on the origin of the double melting peaks. T-M FSC revealed an exothermic process of recrystallization that simultaneously proceeds with melting in the temperature range of the double melting peaks, which starts just above the crystallization temperature.
In the present study, the melting and recrystallization behaviors of PBT in the range of its double melting peaks were examined using both the conventional DSC and FSC with the application of periodic temperature modulation. By changing the heating rate in the range applicable to FSC, we can systematically control the degree of recrystallization and make the melting and subsequent recrystallization more pronounced. On the other hand, PBT crystallizes much faster than PET in orders of magnitude, and the exothermic heat flow can be more pronounced even at slow-scan rates. Therefore, the recrystallization process in the melting region is expected to be observable even under the conventional slow-scan mode.
2. Experimental
PBT was provided by the Chemicals Research Laboratories, Toray Industry, Inc. (Tokyo, Japan) The average molecular weight is
with
. The same PBT was examined in prior papers [
20,
21] in terms of the crystallization and melting kinetics by FSC. The FSC was of Flash DSC 1 (Mettler-Toledo, Greifensee, Switzerland) with a UFS1 chip sensor, and the conventional DSC was of Q100 (TA Instruments, New Castle, DE, USA), both with a refrigerated cooling system. Dry nitrogen gas was purged through the cells at flow rates of 30 and 50 mL min
−1. Temperature calibration was performed with indium. For the FSC, 5-μm-thick sections were cut from a pellet using an ultramicrotome (Leica Microsystems, Wetzlar, Germany). Moreover, for isothermal crystallization in the FSC, quenching was done at the rate of
, which was fast enough to prevent crystallization on cooling in the present condition. With the conventional DSC, crystals were formed from the glass, which was prepared by quenching by placing a sample pan of molten PBT directly on an ice crystal.
The heating-only mode of periodic temperature modulation was employed with a periodic and stepwise change in the temperature of Flash DSC 1 (
Figure 3) and a sinusoidal modulation of Q100. The magnitude
and phase angle
of the dynamic heat capacity of the complex quantity of T-M FSC were determined using the Fourier coefficient of the first harmonic of the heat-flow dataset during each isothermal holding, as described in a prior paper [
15]. Those of the T-M DSC were determined using the Q100 instrument. The obtained magnitude
was adjusted by the heat flow at constant heating and cooling outside the melting region. Moreover, a baseline outside of the melting region was subtracted to determine the phase angle
as a response to the transition kinetics. For T-M FSC, PBT samples were examined repeatedly to get the frequency dependence, so that the obtained results are of re-equilibrated PBT in terms of transesterification, which can influence its mean molar mass.
3. Results and Discussion
The T-M FSC result for the melting region of PBT with double melting peaks is shown in
Figure 4, with a mean underlying heating rate
, which was fixed in the T-M FSC shown below, and different modulation periods
in the range of
.
Figure 4a,b show the magnitude
and phase angle
of the effective heat capacity, including the contribution of the transition kinetics. The heat flow (
) was obtained under the same condition without modulation, and it was converted to heat capacity (
) and included in
Figure 4a to show the double melting peaks. In the region of the double melting peaks, the typical characteristics of the T-M scanning calorimetry of polymer crystal melting [
15] are confirmed as follows and as shown schematically in
Figure 5.
Firstly, the broad peak of and the corresponding large positive peak of represent the response of the melting kinetics. Both and showed a strong dependence on the applied period of the temperature modulation, which is characterized by a time constant of the melting kinetics. For longer , became larger than , suggesting an exothermic process of recrystallization, which proceeds simultaneously with melting.
Secondly, the broad dip in
with a longer
(
Figure 4b) confirms the response of recrystallization, which is characterized by the unusual behavior of a negative peak of
. The appearance of the recrystallization kinetics in
with a longer
is attributed to the difference in the time constants of the kinetics of melting and recrystallization. With a much shorter melting time constant and a longer
, melting follows temperature modulation without delay; therefore, the contribution of the melting kinetics in
approaches zero in an asymptotic manner, as seen in Equation (1). Consequently, with
long enough, the phase angle
is solely determined by the response of recrystallization kinetics which has its time constant longer than
. As shown in the schematic drawing of
Figure 2 and
Figure 5, the response of recrystallization kinetics brings the negative sign of
because the exothermic kinetics becomes faster at higher temperatures [
15]. Therefore, both of the
larger than
in
Figure 4a and the negative peak of
in
Figure 4b confirm that the recrystallization process occurred simultaneously with crystal melting of the PBT.
Figure 6 shows another T-M FSC result of the PBT subjected to quenching. On heating,
in
Figure 6a shows the glass transition, cold crystallization, and melting in that order. Here, the glass transition is characterized by a stepwise increase in
and a positive peak of
with an exothermic peak of enthalpy relaxation in
. Conversely, cold crystallization is indicated by a small drop in
and a dip in
, which is also a characteristic of recrystallization, as mentioned above. Thereafter, a broad dip in
is discernable with the longest
, and it became deeper at higher temperatures, indicating more pronounced recrystallization at higher temperatures. With the contribution of the melting kinetics,
with longer
became larger than
, which suggests simultaneous exothermic process of recrystallization over the temperature range much broader than that in
Figure 4. The frequency dependence is similar to that in
Figure 4.
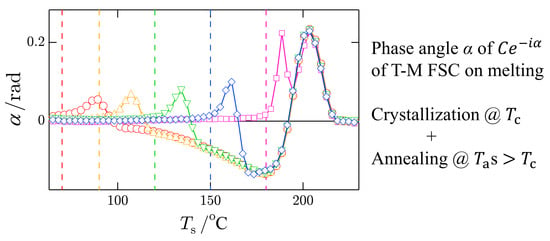
The final T-M FSC result is shown in
Figure 7 with the results of high-temperature annealing of crystals formed at the low temperature of
°C. The temperature jump for subsequent annealing occurred at
. The melting–recrystallization process up to the annealing temperature
could have completed with this annealing process. The results shown in
Figure 7 were obtained with the longest
, and they show that both
and
are on the respective baselines up to
, followed by pure melting in a small temperature window above
. This behavior confirms the appropriate choice of the baseline for
and that the dip in
is real.
Figure 8 shows the corresponding results of T-M DSC obtained at a much slower underlying heating rate
of
. The melting is of crystals formed from the glass heated to
°C with and without subsequent annealing at
°C, which was achieved at a heating scan of
. The trends are similar to those in
Figure 7. This result of PBT is in contrast to that of PET obtained in a previous study [
15]. For PET, the response of recrystallization in
and
of T-M DSC was very weak; however, the final melting peak observed in
shifted to higher temperatures similar that of PBT. Hence, it was concluded that for PET, at heating rates low enough, the shift at low temperatures could be a result of reorganization rather than the melting–recrystallization process. In other words, reorganization exhibits heat flows negligibly smaller than those of melting and recrystallization proceeding as separate processes. In the case of PBT, the recrystallization was pronounced already at low temperatures as evidenced by
and
in
Figure 8, and it was more pronounced at higher temperatures, which is similar to the T-M FSC results shown in
Figure 7. It is inferred that the faster crystallization of PBT than PET at low temperatures also promoted recrystallization. The rate became higher at higher temperatures in accordance with the negative peak of
in the T-M FSC and DSC; the negative peak indicates higher rates at higher temperatures of exothermic kinetics of recrystallization.