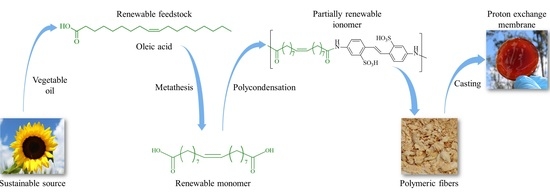
Synthesis and Characterization of Partially Renewable Oleic Acid-Based Ionomers for Proton Exchange Membranes
Abstract
:1. Introduction
2. Experimental Part
2.1. Characterization Techniques
2.2. Reagents
2.3. Synthesis and Characterization of Monomer DA
2.4. Synthesis of the Polyamide Series
2.4.1. Characterization of Polymer DAFA
2.4.2. Characterization of Polymer DAFASA1/4
2.4.3. Characterization of Polymer DAFASA2/4
2.4.4. Characterization of Polymer DAFASA3/4
2.4.5. Characterization of Polymer DASA
2.5. Membrane Preparation, Ion Exchange Capacity, and Water Uptake
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noordover, B.A.J. Polyesters, Polycarbonates and Polyamides Based on Renewable Resources. In Renewable Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 305–354. [Google Scholar]
- Mathers, R.T.; Meier, M.A.R. Green Polymerization Methods: Renewable Starting Materials, Catalysis and Waste Reduction; Wiley-VCH: Weinheim, Germany, 2011; ISBN 9783527326259. [Google Scholar]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Riepl, H.M.; Pettrak, J.; Faulstich, M.; Herrmann, W.A. Self Metathesis of Fatty Alcohols and Amines to Provide Monomers for Polyester and Polyamide Products. Macromol. Symp. 2010, 293, 39–42. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Fernandes, H.; Filgueiras, J.G.; de Azevedo, E.R.; Lima-Neto, B.S. Real time monitoring by time-domain NMR of ring opening metathesis copolymerization of norbornene-based red palm olein monomer with norbornene. Eur. Polym. J. 2020, 110048. [Google Scholar] [CrossRef]
- Luo, X.M.; Ren, L.F.; Zhang, X.L.; Qiang, T.T. The synthesis of oleic acid polyglycol ester catalyzed by solid super acid. J. Surfactants Deterg. 2009, 12, 1–5. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Heise, A. Synthesis of mechanically robust renewable poly(ester-amide)s through co-polymerisation of unsaturated polyesters and synthetic polypeptides. Eur. Polym. J. 2020, 123, 109417. [Google Scholar] [CrossRef]
- Wang, M.; Chen, M.; Fang, Y.; Tan, T. Highly efficient conversion of plant oil to bio-aviation fuel and valuable chemicals by combination of enzymatic transesterification, olefin cross-metathesis, and hydrotreating. Biotechnol. Biofuels 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Velayutham, T.S.; Majid, W.H.A.; Ahmad, A.B.; Kang, G.Y.; Gan, S.N. Synthesis and characterization of polyurethane coatings derived from polyols synthesized with glycerol, phthalic anhydride and oleic acid. Prog. Org. Coatings 2009, 66, 367–371. [Google Scholar] [CrossRef]
- Nguyen, T.H.N.; Balligand, F.; Bormann, A.; Bennevault, V.; Guégan, P. Synthesis of new biobased linear poly(ester amide)s. Eur. Polym. J. 2019, 121, 109314. [Google Scholar] [CrossRef]
- Bansal, K.K.; Upadhyay, P.K.; Kakde, D.; Rosenholm, J.M.; Rosling, A. Synthesis of polyester from renewable feedstock: A comparison between microwave and conventional heating. Mendeleev Commun. 2019, 29, 178–180. [Google Scholar] [CrossRef]
- Yelchuri, V.; Srikanth, K.; Prasad, R.B.N.; Karuna, M.S.L. Olefin metathesis of fatty acids and vegetable oils. J. Chem. Sci. 2019, 131, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Mol, J.C. Catalytic metathesis of unsaturated fatty acid esters and oils. Top. Catal. 2004, 27, 97–104. [Google Scholar] [CrossRef]
- Bauwelinck, J.; Wijnants, M.; Tavernier, S.; Cornet, I. The evaluation of oleic acid alternatives for the biochemical production of 9-octadecenedioic acid. Biochem. Eng. J. 2020, 161, 107660. [Google Scholar] [CrossRef]
- Mudiyanselage, A.Y.; Viamajala, S.; Varanasi, S.; Yamamoto, K. Simple ring-closing metathesis approach for synthesis of PA11, 12, and 13 precursors from oleic acid. ACS Sustain. Chem. Eng. 2014, 2, 2831–2836. [Google Scholar] [CrossRef]
- Firdaus, M.; Meier, M.A.R. Renewable co-polymers derived from vanillin and fatty acid derivatives. Eur. Polym. J. 2013, 49, 156–166. [Google Scholar] [CrossRef]
- Tao, L.; Liu, K.; Li, T.; Xiao, R. Preparation and properties of biobased polyamides based on 1,9-azelaic acid and different chain length diamines. Polym. Bull. 2020, 77, 1135–1156. [Google Scholar] [CrossRef]
- Tao, L.; Liu, K.; Li, T.; Xiao, R. Structure and properties of bio-based polyamide 109 treated with superheated water. Polym. Int. 2019, 68, 1430–1440. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Mohanapriya, S.; Rambabu, G.; Bhat, S.D.; Raj, V. Pectin based nanocomposite membranes as green electrolytes for direct methanol fuel cells. Arab. J. Chem. 2020, 13, 2024–2040. [Google Scholar] [CrossRef]
- Tsai, R.Y.; Chen, P.W.; Kuo, T.Y.; Lin, C.M.; Wang, D.M.; Hsien, T.Y.; Hsieh, H.J. Chitosan/pectin/gum Arabic polyelectrolyte complex: Process-dependent appearance, microstructure analysis and its application. Carbohydr. Polym. 2014, 101, 752–759. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Teruel-Juanes, R.; Arenga, F.; Salaberria, A.M.; Baschetti, M.G.; Labidi, J.; Badia, J.D.; Ribes-Greus, A. Crosslinked chitosan/poly(vinyl alcohol)-based polyelectrolytes for proton exchange membranes. React. Funct. Polym. 2019, 142, 213–222. [Google Scholar] [CrossRef]
- Pasini Cabello, S.D.; Ochoa, N.A.; Takara, E.A.; Mollá, S.; Compañ, V. Influence of Pectin as a green polymer electrolyte on the transport properties of Chitosan-Pectin membranes. Carbohydr. Polym. 2017, 157, 1759–1768. [Google Scholar] [CrossRef] [Green Version]
- Muthumeenal, A.; Neelakandan, S.; Kanagaraj, P.; Nagendran, A. Synthesis and properties of novel proton exchange membranes based on sulfonated polyethersulfone and N-phthaloyl chitosan blends for DMFC applications. Renew. Energy 2016, 86, 922–929. [Google Scholar] [CrossRef]
- Marciel, A.B.; Chung, E.J.; Brettmann, B.K.; Leon, L. Bulk and nanoscale polypeptide based polyelectrolyte complexes. Adv. Colloid Interface Sci. 2017, 239, 187–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Lu, W.; Cai, J.; Hou, Y.; Ouyang, S.; Xie, W.; Gross, R.A. Poly(oleic diacid-co-glycerol): Comparison of polymer structure resulting from chemical and lipase catalysis. Macromolecules 2011, 44, 1977–1985. [Google Scholar] [CrossRef]
- Santiago, A.A.; Ibarra-Palos, A.; Cruz-Morales, J.A.; Sierra, J.M.; Abatal, M.; Alfonso, I.; Vargas, J. Synthesis, characterization, and heavy metal adsorption properties of sulfonated aromatic polyamides. High Perform. Polym. 2018, 30, 591–601. [Google Scholar] [CrossRef]
- Ngo, H.L.; Jones, K.; Foglia, T.A. Metathesis of unsaturated fatty acids: Synthesis of long-chain unsaturated-α,ω-dicarboxylic acids. J. Am. Oil Chem. Soc. 2006, 83, 629–634. [Google Scholar] [CrossRef]
- Niaounakis, M. Recycling of biopolymers—The patent perspective. Eur. Polym. J. 2019, 114, 464–475. [Google Scholar] [CrossRef]
- Bhadani, A.; Iwabata, K.; Sakai, K.; Koura, S.; Sakai, H.; Abe, M. Sustainable oleic and stearic acid based biodegradable surfactants. RSC Adv. 2017, 7, 10433–10442. [Google Scholar] [CrossRef] [Green Version]
- Vargas, J.; Santiago, A.A.; Tlenkopatchev, M.A.; Gaviño, R.; Laguna, M.F.; López-González, M.; Riande, E. Gas transport and ionic transport in membranes based on polynorbornenes with functionalized imide side groups. Macromolecules 2007, 40, 563–570. [Google Scholar] [CrossRef]
- Aranda-Suárez, I.; Corona-García, C.; Santiago, A.A.; López Morales, S.; Abatal, M.; López-González, M.; Vargas, J. Synthesis and Gas Permeability of Chemically Cross-Linked Polynorbornene Dicarboximides Bearing Fluorinated Moieties. Macromol. Chem. Phys. 2019, 220, 1800481. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Lee, K.H.; Chu, J.Y.; Ryu, S.K.; Kim, H.G.; Lee, J.Y.; Lee, H.K.; Yoo, D.J. Ameliorated performance of sulfonated poly(arylene ether sulfone) block copolymers with increased hydrophilic oligomer ratio in proton-exchange membrane fuel cells operating at 80% relative humidity. Polymers (Basel) 2020, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. Physical Properties of Molecular Crystals, Liquids, and Glasses, 2nd ed.; Wiley: Hoboken, NJ, USA, 1968; ISBN 9780471087663. [Google Scholar]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers; Elsevier Inc.: Amsterdam, The Netherlands, 2009; ISBN 9780080548197. [Google Scholar]
- Pérez-Padilla, Y.; Smit, M.A.; Aguilar-Vega, M.J. Preparation and Characterization of Sulfonated Copolyamides Based on Poly(hexafluoroisopropylidene) Isophthalamides for Polymer Electrolytic Membranes. Ind. Eng. Chem. Res. 2011, 50, 9617–9624. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Park, C.J.; Yoo, D.J. Alleviating the mechanical and thermal degradations of highly sulfonated Poly(ether ether ketone) blocks via copolymerization with hydrophobic unit for intermediate humidity fuel cells. Polymers 2018, 10, 1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiago, A.A.; Vargas, J.; Cruz-Gómez, J.; Tlenkopatchev, M.A.; Gaviño, R.; López-González, M.; Riande, E. Synthesis and ionic transport of sulfonated ring-opened polynorbornene based copolymers. Polymer (Guildf) 2011, 52, 4208–4220. [Google Scholar] [CrossRef]
- Mokrini, A.; Acosta, J.L. Studies of sulfonated block copolymer and its blends. Polymer (Guildf) 2001, 42, 9–15. [Google Scholar] [CrossRef]
- Genies, C.; Mercier, R.; Sillion, B.; Cornet, N.; Gebel, G.; Pineri, M. Soluble sulfonated naphthalenic polyimides as materials for proton exchange membranes. Polymer (Guildf) 2001, 42, 359–373. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Solid polymer electrolyte membranes for fuel cell applications—A review. J. Memb. Sci. 2005, 259, 10–26. [Google Scholar] [CrossRef]










| Polymer | DA a [n+m, mmol] | FA b [n, mmol)] | SA c [m, mmol] |
|---|---|---|---|
| DAFA | 0.64 | 0.64 | 0 |
| DAFASA1/4 | 0.64 | 0.48 | 0.16 |
| DAFASA2/4 | 0.64 | 0.32 | 0.32 |
| DAFASA3/4 | 0.64 | 0.16 | 0.48 |
| DASA | 0.64 | 0 | 0.64 |
| Polymer | Tg [°C] a | Td [°C] b | DC [%] c | dinterchain [Å] d | ρ [g cm−3] e | FFV [%] f | ηinh [dL g−1] g |
|---|---|---|---|---|---|---|---|
| DAFA | 105 h/131 i | 456 | 5.3 | 4.93 | 1.271 | 13.7 | 0.374 |
| DAFASA1/4 | 103 h/175 i | 155 j/385 k | ND l | 4.56 | 1.241 | 15.7 | 0.369 |
| DAFASA2/4 | 100 h/248 i | 176 j/388 k | ND l | 4.38 | 1.247 | 15.2 | 0.361 |
| DAFASA3/4 | 96 h/286 i | 194 j/406 k | ND l | 4.25 | 1.249 | 15.1 | 0.356 |
| DASA | 96 h/319 i | 197 j/423 k | 12.8 | 4.1 | 1.245 | 15.3 | 0.336 |
| Polymer | DS [%] a | WU [%] b | IECTheo [meq g−1] c | IEC [meq g−1] d | σ Hydrated [mS cm−1] e | σ Activated [mS cm−1] e |
|---|---|---|---|---|---|---|
| DAFA | 0 | 1.9 | NA f | NA f | NA f | NA f |
| DAFASA1/4 | 23.7 | 9.7 | 0.63 | 0.482 | 0.035 | 0.34 |
| DAFASA2/4 | 47.3 | 17.1 | 1.321 | 0.832 | 0.407 | 1.552 |
| DAFASA3/4 | 71.6 | 36.4 | 2.084 | 1.816 | 0.449 | Pending g |
| DASA | 100 | 44.1 | 2.929 | 2.287 | 0.594 | Pending g |
| Polymer | IEC Experimental [meq g−1] a | WU [%] b | σ [mS cm−1] c | Reference Author |
|---|---|---|---|---|
| C5FNDDIH5 d | 0.3 | 37.3 | 0.1 | [39] |
| Poly-SHPhNDI e | 0.8 | 12.4 | 0.4 | [32] |
| DASA | 2.2 | 44.1 | 0.5 | This study |
| CH3 5 30/70 f | 0.9 | 19 | 1.3 | [40] |
| DAFASA2/4 | 0.8 | 17.1 | 1.5 | This study |
| CF3 5 30/70 g | 0.8 | 14 | 1.7 | [41] |
| HFAS55 h | 1.6 | 22.5 | 3.3 | [37] |
| CMV i | 2.4 | 25 | 5.1 | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona-García, C.; Onchi, A.; Santiago, A.A.; Martínez, A.; Pacheco-Catalán, D.E.; Alfonso, I.; Vargas, J. Synthesis and Characterization of Partially Renewable Oleic Acid-Based Ionomers for Proton Exchange Membranes. Polymers 2021, 13, 130. https://doi.org/10.3390/polym13010130
Corona-García C, Onchi A, Santiago AA, Martínez A, Pacheco-Catalán DE, Alfonso I, Vargas J. Synthesis and Characterization of Partially Renewable Oleic Acid-Based Ionomers for Proton Exchange Membranes. Polymers. 2021; 13(1):130. https://doi.org/10.3390/polym13010130
Chicago/Turabian StyleCorona-García, Carlos, Alejandro Onchi, Arlette A. Santiago, Araceli Martínez, Daniella Esperanza Pacheco-Catalán, Ismeli Alfonso, and Joel Vargas. 2021. "Synthesis and Characterization of Partially Renewable Oleic Acid-Based Ionomers for Proton Exchange Membranes" Polymers 13, no. 1: 130. https://doi.org/10.3390/polym13010130