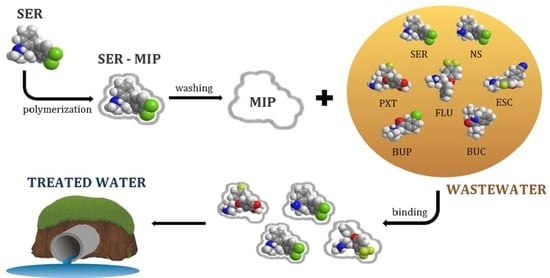
Molecularly Imprinted Polymers for the Removal of Antide-Pressants from Contaminated Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Synthesis of MIP
2.2. Selection of the Material: Batch Rebinding
2.3. Reusability Experiments
2.4. Cross-Reactivity Experiments
2.5. Time to Reach Equilibrium
2.6. Binding in WW Matrix: Influence of pH, Salts, and Chemical Oxygen Demand
2.7. Upscale Experiment
2.8. Leaching Evaluation
2.9. Chemical and Morphological Characterization
2.10. HPLC Measurements
3. Results and Discussion
3.1. MIP Synthesis, Selection, and Reusability
3.2. Cross-Reactivity
3.3. Time to Reach the Equilibrium
3.4. Effect of WW Matrix
3.5. Upscale Experiment
3.6. Leaching
3.7. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Sarpong, K.A.; Xu, W.; Huang, W.; Yang, W. The Development of Molecularly Imprinted Polymers in the Clean-Up of Water Pollutants: A Review. Am. J. Anal. Chem. 2019, 10, 202–226. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Oliván, L.M. The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2019; Volume 66. [Google Scholar]
- Bauer, M.; Monz, B.U.; Montejo, A.L.; Quail, D.; Dantchev, N.; Demyttenaere, K.; Garcia-Cebrian, A.; Grassi, L.; Perahia, D.G.; Reed, C.; et al. Prescribing patterns of antidepressants in Europe: Results from the Factors Influencing Depression Endpoints Research (FINDER) study. Eur. Psychiatry 2008, 23, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, A.V.; Pineda, M.D.; Venkata, K.C.N. Comprehension of Top 200 Prescribed Drugs in the US as a Resource for Pharmacy Teaching, Training and Practice. Pharmacy 2018, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- NIJZ Poraba Ambulantno Predpisanih Zdravil v Sloveniji v Letu 2019. Available online: https://www.nijz.si/sites/www.nijz.si/files/publikacije-datoteke/publikacija_220520_koncno_0.pdf (accessed on 22 November 2020).
- Arnnok, P.; Singh, R.R.; Burakham, R.; Pérez-Fuentetaja, A.; Aga, D.S. Selective Uptake and Bioaccumulation of Antidepressants in Fish from Effluent-Impacted Niagara River. Environ. Sci. Technol. 2017, 51, 10652–10662. [Google Scholar] [CrossRef]
- Bergersen, O.; Hanssen, K.Ø.; Vasskog, T. Anaerobic treatment of sewage sludge containing selective serotonin reuptake inhibitors. Bioresour. Technol. 2012, 117, 325–332. [Google Scholar] [CrossRef]
- Gornik, T.; Vozic, A.; Heath, E.; Trontelj, J.; Roskar, R.; Zigon, D.; Vione, D.; Kosjek, T. Determination and photodegradation of sertraline residues in aqueous environment. Environ. Pollut. 2020, 256, 113431. [Google Scholar] [CrossRef]
- Gornik, T.; Kovacic, A.; Heath, E.; Hollender, J.; Kosjek, T. Biotransformation study of antidepressant sertraline and its removal during biological wastewater treatment. Water Res. 2020, 181, 115864. [Google Scholar] [CrossRef]
- Mole, R.A.; Brooks, B. Global scanning of selective serotonin reuptake inhibitors: Occurrence, wastewater treatment and hazards in aquatic systems. Environ. Pollut. 2019, 250, 1019–1031. [Google Scholar] [CrossRef]
- Schultz, M.M.; Furlong, E.T.; Kolpin, D.W.; Werner, S.L.; Schoenfuss, H.L.; Barber, L.B.; Blazer, V.S.; Norris, D.O.; Vajda, A.M. Antidepressant Pharmaceuticals in Two U.S. Effluent-Impacted Streams: Occurrence and Fate in Water and Sediment, and Selective Uptake in Fish Neural Tissue. Environ. Sci. Technol. 2010, 44, 1918–1925. [Google Scholar] [CrossRef]
- OECD. Pharmaceutical Residues in Freshwater: Hazards and Policy Responses-Policy Highlights. In OECD Studies on Water; OECD Publishing: Paris, France, 2019; Volume 2019. [Google Scholar]
- Park, J.-W.; Heah, T.P.; Gouffon, J.S.; Henry, T.; Sayler, G.S. Global gene expression in larval zebrafish (Danio rerio) exposed to selective serotonin reuptake inhibitors (fluoxetine and sertraline) reveals unique expression profiles and potential biomarkers of exposure. Environ. Pollut. 2012, 167, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, J.; Sehonova, P.; Hodkovicova, N.; Vecerkova, L.; Blahova, J.; Franc, A.; Marsalek, P.; Mares, J.; Tichy, F.; Svobodova, Z.; et al. The effect of foodborne sertraline on rainbow trout (Oncorhynchus mykiss). Sci. Total. Environ. 2020, 708, 135082. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Li, S.; Nie, Y.; Ma, B.; Liu, J. Behavioral and biochemical responses in freshwater fish Carassius auratus exposed to sertraline. Chemosphere 2015, 135, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.J.; Pereira, A.M.; Meisel, L.M.; Lino, C.; Pena, A. Reviewing the serotonin reuptake inhibitors (SSRIs) footprint in the aquatic biota: Uptake, bioaccumulation and ecotoxicology. Environ. Pollut. 2015, 197, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Marques, R.; Noronha, J.; Mexia, J.; Carvalho, G.; Oehmen, A.; Reis, M. Assessing the diurnal variability of pharmaceutical and personal care products in a full-scale activated sludge plant. Environ. Pollut. 2011, 159, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Fu, B.; Zhang, T.; Wu, Y.; Zhou, Z.; Zhao, J.; Yang, E.; Qian, T.; Luo, J. Fate of typical endocrine active compounds in full-scale wastewater treatment plants: Distribution, removal efficiency and potential risks. Bioresour. Technol. 2020, 310, 123436. [Google Scholar] [CrossRef]
- Ek, M.; Baresel, C.; Magnér, J.; Bergström, R.; Harding, M. Activated carbon for the removal of pharmaceutical residues from treated wastewater. Water Sci. Technol. 2014, 69, 2372–2380. [Google Scholar] [CrossRef] [Green Version]
- Guillossou, R.; Le Roux, J.; Mailler, R.; Vulliet, E.; Morlay, C.; Nauleau, F.; Gasperi, J.; Rocher, V. Organic micropollutants in a large wastewater treatment plant: What are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere 2019, 218, 1050–1060. [Google Scholar] [CrossRef]
- Kårelid, V.; Larsson, G.; Björlenius, B. Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. J. Environ. Manag. 2017, 193, 491–502. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Silva, B.; Martins, M.; Rosca, M.; Rocha, V.; Lago, A.; Neves, I.C.; Tavares, T. Waste-based biosorbents as cost-effective alternatives to commercial adsorbents for the retention of fluoxetine from water. Sep. Purif. Technol. 2020, 235, 116139. [Google Scholar] [CrossRef] [Green Version]
- Jaria, G.; Calisto, V.; Gil, M.V.; Otero, M.; Esteves, V.I. Removal of fluoxetine from water by adsorbent materials produced from paper mill sludge. J. Colloid Interface Sci. 2015, 448, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Orooji, Y.; Alizadeh, A. Applying Membrane Distillation for the Recovery of Nitrate from Saline Water Using PVDF Membranes Modified as Superhydrophobic Membranes. Polymers 2020, 12, 2774. [Google Scholar] [CrossRef] [PubMed]
- Orooji, Y.; Jaleh, B.; Homayouni, F.; Fakhri, P.; Kashfi, M.; Torkamany, M.J.; Yousefi, A.A. Laser Ablation-Assisted Synthesis of Poly (Vinylidene Fluoride)/Au Nanocomposites: Crystalline Phase and Micromechanical Finite Element Analysis. Polymers 2020, 12, 2630. [Google Scholar] [CrossRef] [PubMed]
- Mashile, G.P.; Dimpe, K.M.; Nomngongo, P.N. A Biodegradable Magnetic Nanocomposite as a Superabsorbent for the Simultaneous Removal of Selected Fluoroquinolones from Environmental Water Matrices: Isotherm, Kinetics, Thermodynamic Studies and Cost Analysis. Polymers 2020, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Awual, R. A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Cantarella, M.; Carroccio, S.C.; Dattilo, S.; Avolio, R.; Castaldo, R.; Puglisi, C.; Privitera, V. Molecularly imprinted polymer for selective adsorption of diclofenac from contaminated water. Chem. Eng. J. 2019, 367, 180–188. [Google Scholar] [CrossRef]
- Qiu, L.; Jaria, G.; Gil, M.; Feng, J.; Dai, Y.; Esteves, V.I.; Otero, M.A.; Calisto, V. Core−Shell Molecularly Imprinted Polymers on Magnetic Yeast for the Removal of Sulfamethoxazole from Water. Polymers 2020, 12, 1385. [Google Scholar] [CrossRef]
- Sellergren, B. Molecularly Imprinted Polymers: Man-Made Mimics of Antibodies and Their Application in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Affinisep Affinimip. Available online: https://www.affinisep.com/spe-kits-applications/spe-kit-for-sample-preparation/affinimip-spe-selectives-mip-spe-cartridges (accessed on 3 August 2020).
- SupelMIP® SPE, Supelco. Available online: https://www.sigmaaldrich.com/analytical-chromatography/sample-preparation/spe/supelmip.html (accessed on 3 August 2020).
- Mattiasson, B.; Ye, L. Molecularly Imprinted Polymers in Biotechnology (Advances in Biochemical Engineering/Biotechnology); Springer International Publishing: Cham, Switzerland, 2015; Volume 150. [Google Scholar]
- Sellergren, B.; Allender, C.J. Molecularly imprinted polymers: A bridge to advanced drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1733–1741. [Google Scholar] [CrossRef]
- Altintas, Z.; Chianella, I.; Da Ponte, G.; Paulussen, S.; Gaeta, S.; Tothill, I. Development of functionalized nanostructured polymeric membranes for water purification. Chem. Eng. J. 2016, 300, 358–366. [Google Scholar] [CrossRef]
- An, F.-Q.; Li, H.-F.; Guo, X.-D.; Hu, T.-P.; Gao, B.; Gao, J.-F. Design of novel “imprinting synchronized with crosslinking” surface imprinted technique and its application for selectively removing phenols from aqueous solution. Eur. Polym. J. 2019, 112, 273–282. [Google Scholar] [CrossRef]
- Le Noir, M.; Lepeuple, A.-S.; Guieysse, B.; Mattiasson, B. Selective removal of 17β-estradiol at trace concentration using a molecularly imprinted polymer. Water Res. 2007, 41, 2825–2831. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Mao, J.H.; Zhang, W.; Wang, C.; Cao, M.; Wang, X.D.; Wang, K.Y.; Xiong, X. A novel strategy for selective removal and rapid collection of triclosan from aquatic environment using magnetic molecularly imprinted nano−polymers. Chemosphere 2020, 238, 124640. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Sun, D.; Gao, J.; Zhao, Q.; Wang, X.; Teng, F.; Quan, X.; Chen, J. Preparation of molecularly imprinted polymer nanoparticles for selective removal of fluoroquinolone antibiotics in aqueous solution. J. Hazard. Mater. 2013, 750–757. [Google Scholar] [CrossRef]
- DeVane, C.L.; Liston, H.L.; Markowitz, J.S.; De Vane, C.L. Clinical Pharmacokinetics of Sertraline. Clin. Pharmacokinet. 2002, 41, 1247–1266. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E.; Kompare, B. Removal of pharmaceutical residues in a pilot wastewater treatment plant. Anal. Bioanal. Chem. 2007, 387, 1379–1387. [Google Scholar] [CrossRef]
- Abdouss, M.; Asadi, E.; Azodi-Deilami, S.; Beik-Mohammadi, N.; Aslanzadeh, S.A. Development and characterization of molecularly imprinted polymers for controlled release of citalopram. J. Mater. Sci. Mater. Electron. 2011, 22, 2273–2281. [Google Scholar] [CrossRef]
- Chapuis, F.; Mullot, J.-U.; Pichon, V.; Tuffal, G.; Hennion, M.-C. Molecularly imprinted polymers for the clean-up of a basic drug from environmental and biological samples. J. Chromatogr. A 2006, 1135, 127–134. [Google Scholar] [CrossRef]
- Kempe, H.; Kempe, M. Influence of salt ions on binding to molecularly imprinted polymers. Anal. Bioanal. Chem. 2009, 396, 1599–1606. [Google Scholar] [CrossRef] [Green Version]
- Horemans, F.; Weustenraed, A.; Spivak, D.A.; Cleij, T.J. Towards water compatible MIPs for sensing in aqueous media. J. Mol. Recognit. 2012, 25, 344–351. [Google Scholar] [CrossRef]
- Dirion, B.; Cobb, Z.; Schillinger, E.; Andersson, A.L.I.; Sellergren, B. Water-Compatible Molecularly Imprinted Polymers Obtained via High-Throughput Synthesis and Experimental Design. J. Am. Chem. Soc. 2003, 125, 15101–15109. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Row, K.H. Characteristic and Synthetic Approach of Molecularly Imprinted Polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef] [Green Version]
- Arvand, M.; Hashemi, M. Synthesis by precipitation polymerization of a molecularly imprinted polymer membrane for the potentiometric determination of sertraline in tablets and biological fluids. J. Braz. Chem. Soc. 2012, 23, 392–402. [Google Scholar] [CrossRef]
- Khalilian, F.; Kermani, F.K. Selective Dispersive Solid Phase Extraction of Ser-Traline Using Surface Molecu-larly Imprinted Polymer Grafted on SiO2/Graphene Oxide. J. Chem. Health Risks 2017, 7, 49–60. [Google Scholar]
- Krupadam, R.J.; Patel, G.P.; Balasubramanian, R. Removal of cyanotoxins from surface water resources using reusable molecularly imprinted polymer adsorbents. Environ. Sci. Pollut. Res. 2011, 19, 1841–1851. [Google Scholar] [CrossRef]
- Krupadam, R.J.; Khan, M.S.; Wate, S.R. Removal of probable human carcinogenic polycyclic aromatic hydrocarbons from contaminated water using molecularly imprinted polymer. Water Res. 2010, 44, 681–688. [Google Scholar] [CrossRef]
- Krupadam, R.J.; Bhagat, B.; Wate, S.R.; Bodhe, G.L.; Sellergren, B.; Anjaneyulu, Y. Fluorescence Spectrophotometer Analysis of Polycyclic Aromatic Hydrocarbons in Environmental Samples Based on Solid Phase Extraction Using Molecularly Imprinted Polymer. Environ. Sci. Technol. 2009, 43, 2871–2877. [Google Scholar] [CrossRef]
- Murray, A.; Örmeci, B. Application of molecularly imprinted and non-imprinted polymers for removal of emerging contaminants in water and wastewater treatment: A review. Environ. Sci. Pollut. Res. 2012, 19, 3820–3830. [Google Scholar] [CrossRef]
- Tadeo, X.; López-Méndez, B.; Castaño, D.; Trigueros, T.; Millet, O. Protein Stabilization and the Hofmeister Effect: The Role of Hydrophobic Solvation. Biophys. J. 2009, 97, 2595–2603. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Yang, S.; Wang, P.G. Matrix effects and application of matrix effect factor. Bioanalysis 2017, 9, 1839–1844. [Google Scholar] [CrossRef] [Green Version]
- Azizi, A.; Bottaro, C.S. A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J. Chromatogr. A 2020, 1614, 460603. [Google Scholar] [CrossRef] [PubMed]
- Martín-Esteban, A.; Sellergren, B. 2.17-Molecularly Imprinted Polymers. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 331–344. [Google Scholar]
- Sellergren, B.; Lanza, F. Techniques and Instrumentation in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2001; Volume 23, pp. 355–375. [Google Scholar]
- Turiel, E.; Esteban, A.M. 8-Molecularly imprinted polymers. In Solid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 215–233. [Google Scholar]
- Ellwanger, A.; Karlsson, L.; Owens, P.K.; Berggren, C.; Crecenzi, C.; Ensing, K.; Bayoudh, S.; Cormack, P.A.; Sherrington, D.; Sellergren, B. Evaluation of methods aimed at complete removal of template from molecularly imprinted polymers. Analyst 2001, 126, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, R.A.; Carro, A.; Alvarez-Lorenzo, C.; Concheiro, A. To Remove or Not to Remove? The Challenge of Extracting the Template to Make the Cavities Available in Molecularly Imprinted Polymers (MIPs). Int. J. Mol. Sci. 2011, 12, 4327–4347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Activated Charcoal C3345. Available online: https://www.sigmaaldrich.com/catalog/product/sigald/c3345 (accessed on 8 September 2020).








| Material | Template | MAA | mMA | HEMA | EGDMA | Initiator (V-65) | Porogen |
|---|---|---|---|---|---|---|---|
| MIP1 | SER×HCl (1) | 4 | / | / | 20 | 1 wt % based on total monomers | CHCl3 |
| MIP2 | SER×HCl (1) | 4 | 8 | / | 12 | CHCl3 | |
| MIP3 | SER×HCl (1) | 4 | / | 8 | 12 | CHCl3 | |
| MIP4 | SER (1) | 4 | / | / | 20 | MeOH | |
| MIP5 | SER (1) | 4 | / | / | 20 | CHCl3 | |
| MIP6 | SER (1) | 4 | / | / | 20 | ACN | |
| MIP7 | SER (1) | 4 | / | / | 20 | toluen | |
| MIP8 | SER (1) | 4 | 8 | / | 12 | CHCl3 | |
| MIP9 | SER (1) | 4 | 8 | / | 12 | ACN | |
| MIP10 | SER (1) | 4 | 8 | / | 12 | toluen | |
| MIP11 | SER (1) | 4 | / | 8 | 12 | CHCl3 | |
| MIP12 | SER (1) | 4 | / | 8 | 12 | ACN | |
| MIP13 | SER (1) | 4 | / | 8 | 12 | toluen |
| MIP5 | MIP9 | MIP13 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Capacity (mg·g−1) | Selectivity Factor (α) | % (MIP-NIP) | Capacity (mg·g−1) | Selectivity Factor (α) | % (MIP-NIP) | Capacity (mg·g−1) | Selectivity Factor (α) | % (MIP-NIP) |
| SER | 26.6 ± 0.6 | 1.0 | 8.4 | 24.4 ± 0.4 | 1.0 | 17.4 | 11.0 ± 0.3 | 1.0 | 25.6 |
| NS | 26.3 ± 0.3 | 0.8 | 7.2 | 23.1 ± 0.4 | 1.0 | 17.1 | 13.0 ± 0.1 | 0.7 | 33.5 |
| FLU | 25.3 ± 0.6 | 1.3 | 4.9 | 22.4 ± 0.4 | 1.2 | 16.2 | 10.3 ± 0.2 | 1.1 | 25.3 |
| ESC | 20.3 ± 0.5 | 2.9 | 4.7 | 18.8 ± 0.6 | 2.7 | 14.8 | 5.5 ± 0.2 | 2.8 | 10.8 |
| PXT | 27.3 ± 0.1 | 1.0 | 3.4 | 26.3 ± 0.4 | 1.0 | 16.8 | 11.3 ± 0.1 | 1.1 | 25.0 |
| BUP | 7.9 ± 0.1 | 9.6 | 2.2 | 6.5 ± 0.3 | 9.8 | 8.0 | 3.5 ± 0.3 | 3.3 | 12.3 |
| BUC | 10.3 ± 0.3 | 8.7 | 9.4 | 9.7 ± 0.3 | 7.0 | 9.4 | 2.2 ± 0.4 | 7.0 | 6.1 |
| MIP 5 | % C | % H | NIP 5 | % C | % H |
| Theoretical | 60.21 | 7.11 | Theoretical | 60.21 | 7.11 |
| Actual | 59.56 | 7.81 | Actual | 59.68 | 8 |
| Deviation | 0.65 | −0.7 | Deviation | 0.53 | −0.89 |
| MIP 9 | % C | % H | NIP 9 | % C | % H |
| Theoretical | 59.99 | 7.28 | Theoretical | 59.99 | 7.28 |
| Actual | 59.55 | 8.18 | Actual | 60.2 | 8.25 |
| Deviation | 0.44 | −0.9 | Deviation | −0.21 | −0.97 |
| MIP 13 | % C | % H | NIP 13 | % C | % H |
| Theoretical | 59.27 | 7.23 | Theoretical | 59.27 | 7.23 |
| Actual | 57.00 | 7.60 | Actual | 58.03 | 8.17 |
| Deviation | 2.27 | −0.37 | Deviation | 1.24 | −0.94 |
| Material | BET Area (m2·g−1) | Pore Size (nm) | Pore Volume (cm3·g−1) |
|---|---|---|---|
| MIP5 | 193.8 | 7.7 | 0.374061 |
| NIP5 | 262.1 | 7.2 | 0.470623 |
| MIP9 | 136.0 | 10.3 | 0.349167 |
| NIP9 | 125.7 | 9.6 | 0.300946 |
| MIP13 | 27.4 | 7.6 | 0.051835 |
| NIP13 | 5.5 | 6.6 | 0.009074 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gornik, T.; Shinde, S.; Lamovsek, L.; Koblar, M.; Heath, E.; Sellergren, B.; Kosjek, T. Molecularly Imprinted Polymers for the Removal of Antide-Pressants from Contaminated Wastewater. Polymers 2021, 13, 120. https://doi.org/10.3390/polym13010120
Gornik T, Shinde S, Lamovsek L, Koblar M, Heath E, Sellergren B, Kosjek T. Molecularly Imprinted Polymers for the Removal of Antide-Pressants from Contaminated Wastewater. Polymers. 2021; 13(1):120. https://doi.org/10.3390/polym13010120
Chicago/Turabian StyleGornik, Tjasa, Sudhirkumar Shinde, Lea Lamovsek, Maja Koblar, Ester Heath, Börje Sellergren, and Tina Kosjek. 2021. "Molecularly Imprinted Polymers for the Removal of Antide-Pressants from Contaminated Wastewater" Polymers 13, no. 1: 120. https://doi.org/10.3390/polym13010120