Preparation and Thermomechanical Properties of Ketone Mesogenic Liquid Crystalline Epoxy Resin Composites with Functionalized Boron Nitride
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of 1,5-bis(ρ-glycidyloxy-phenyl)-1,4-pentadiene-3-one (K–LCE)
2.3. Surface Modification of BN Fillers
2.4. Preparation of K–LCE/BN Composites
2.5. Characterization
3. Results and Discussion
3.1. Characterization of BN Surface Modification
3.2. Morphology Observation of K–LCE/BN Composites
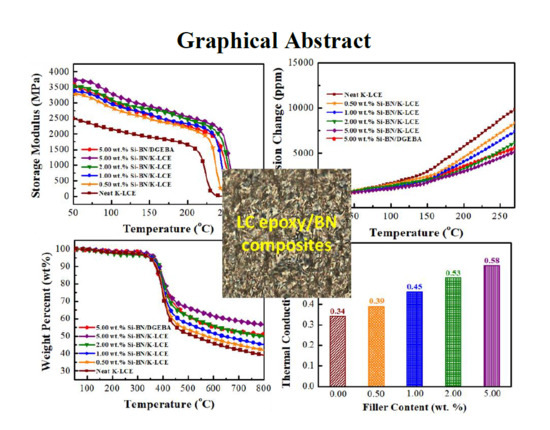
3.3. Thermal and Mechanical Properties of K–LCE/BN Composites
3.4. Thermal Conductivity of K–LCE/BN Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGlen, R.J.; Jachuck, R. Integrated thermal management techniques for high power electronic devices. Appl. Therm. Eng. 2004, 24, 1143–1156. [Google Scholar] [CrossRef]
- Garimella, S.V.; Fleischer, A.S.; Murthy, J.Y.; Keshavarzi, A.; Prasher, R.; Patel, C.; Bhavnani, S.H.; Venkatasubramanian, R.; Mahajan, R.; Joshi, Y.; et al. Thermal Challenges in Next-Generation Electronic Systems. IEEE Trans. Compon. Packag. Technol. 2008, 31, 801–815. [Google Scholar] [CrossRef]
- Liang, Y.C.; Zhang, J.N.; Li, M.; Guo, Y.P.; Yuan, J.S. Thermal Analysis of the Heat Exchanger for Power Electronic Device with Higher Power Density. Prz. Elektrotechniczn 2012, 88, 328–332. [Google Scholar]
- Gonon, P.; Sylvestre, A.; Teysseyre, J.; Prior, C. Dielectric properties of epoxy/silica composites used for microlectronic packaging, and their dependence on post-curing. J. Mater. Sci. Mater. Electron. 2001, 12, 81–86. [Google Scholar] [CrossRef]
- Harada, M.; Okamoto, N.; Kannan, P.; Ochi, M. Fracture toughness and fracture mechanism of liquid-crystalline epoxy resins with different polydomain structures. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 2337–2345. [Google Scholar] [CrossRef]
- Liu, Y.L.; Cai, Z.Q.; Wen, X.; Pi, P.; Zheng, D.; Cheng, J.; Yang, Z. A new and efficient synthetic method of a liquid crystalline epoxy resin with biphenol and aromatic ester group. Polym. Bull. 2011, 67, 57–66. [Google Scholar] [CrossRef]
- Harada, M.; Ando, J.; Ochi, M. Synthesis, Characterization, and Mechanical Properties of a Novel Terphenyl Liquid Crystalline Epoxy Resin. J. Appl. Polym. Sci. 2015, 132, 41296. [Google Scholar] [CrossRef]
- Nazarenko, V.; Kurik, M.V.; Klimusheva, G.V.; Gotra, Z.Y.; Sorokin, V.M.; Lisetski, L.M. Liquid crystals in Ukraine and Ukrainians in liquid crystals. J. Mol. Liq. 2018, 267, 29–33. [Google Scholar] [CrossRef]
- Lin, Y.S.; Hsu, S.L.C.; Ho, T.H.; Cheng, S.S.; Hsiao, Y.H. Synthesis, characterization, and thermomechanical properties of liquid crystalline epoxy resin containing ketone mesogen. Polym. Eng. Sci. 2017, 57, 424–431. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Fu, J.F.; Shi, L.Y.; Zhong, Q.D.; Chen, Y.; Chen, L.Y. Thermally conductive and electrically insulative nanocomposites based on hyperbranched epoxy and nano-Al2O3 particles modified epoxy resin. Polym. Adv. Technol. 2011, 22, 1032–1041. [Google Scholar] [CrossRef]
- Fang, L.; Wu, C.; Qian, R.; Xie, L.; Yang, K.; Jiang, P. Nano–micro structure of functionalized boron nitride and aluminum oxide for epoxy composites with enhanced thermal conductivity and breakdown strength. RSC Adv. 2014, 40, 21010–21017. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; Lv, L.; Pan, Y.; Zhang, C.; He, Y. Fabrication of graphene oxide–alumina hybrids to reinforce the anti-corrosion performance of composite epoxy coatings. Appl. Surf. Sci. 2015, 351, 986–996. [Google Scholar] [CrossRef]
- Akhtar, M.W.; Lee, Y.S.; Yoo, D.J.; Kim, J.S. Alumina-graphene hybrid filled epoxy composite: Quantitative validation and enhanced thermal conductivity. Compos. Part B Eng. 2017, 131, 184–195. [Google Scholar] [CrossRef]
- Lazouziet, G.; Vuksanović, M.M.; Tomić, N.Z.; Mitrić, M.; Petrović, M.; Radojević, V.; Heinemann, R.J. Optimized preparation of alumina based fillers for tuning composite properties. Ceram. Int. 2018, 44, 7442–7449. [Google Scholar] [CrossRef]
- Choi, J.; Yang, S.; Yu, S.; Shin, H.; Cho, M. Method of scale bridging for thermoelasticity of cross-linked epoxy/SiC nanocomposites at a wide range of temperatures. Polymer 2012, 53, 5178–5189. [Google Scholar] [CrossRef]
- Abenojar, J.; Pantoja, M.; Martinez, M.A.; Real, J.C. Aging by moisture and/or temperature of epoxy/SiC composites: Thermal and mechanical properties. J. Compos. Mater. 2015, 49, 2963–2975. [Google Scholar] [CrossRef]
- Shen, D.; Zhan, Z.; Liu, Z.; Cao, Y.; Zhou, L.; Liu, Y.; Dai, W.; Nishimura, K.; Li, C.; Lin, C.T.; et al. Enhanced thermal conductivity of epoxy composites filled with silicon carbide nanowires. Sci. Rep. 2017, 7, 2606. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Fu, R.; Agathopoulos, S.; Gu, X.; Zhao, W. Thermal conductivity and fire resistance of epoxy molding compounds filled with Si3N4 and Al(OH)3. Mater. Design. 2012, 34, 820–824. [Google Scholar] [CrossRef]
- Kusunose, T.; Yagi, T.; Fiorz, S.H.; Sekino, T. Fabrication of epoxy/silicon nitridenanowire composites and evaluation of their thermal conductivity. J. Mater. Chem. A 2013, 1, 3440–3445. [Google Scholar] [CrossRef]
- Shimamura, A.; Hotta, Y.; Hyuga, H.; Kondo, N.; Hirao, K. Effect of amounts and types of silicon nitride on thermal conductivity of Si3N4/epoxy resin composite. J. Ceram. Soc. Jpn. 2015, 123, 908–912. [Google Scholar] [CrossRef] [Green Version]
- Teng, C.C.; Ma, C.C.M.; Chiou, K.C.; Lee, T.M. Synergetic effect of thermal conductive properties of epoxy composites containing functionalized multi-walled carbon nanotubes and aluminum nitride. Compos. Part B Eng. 2012, 43, 265–271. [Google Scholar] [CrossRef]
- Choudhury, M.; Mohanty, S.; Nayak, S.K. Effect of surface modification of aluminum nitride on electrical and thermal characterizations of thermosetting polymeric nanocomposites. Polym. Compos. 2013, 34, 1–14. [Google Scholar] [CrossRef]
- Qian, R.; Yu, J.; Xie, L.; Li, Y.; Jiang, P. Efficient thermal properties enhancement to hyperbranched aromatic polyamide grafted aluminum nitride in epoxy composites. Polym. Adv. Technol. 2013, 24, 348–356. [Google Scholar] [CrossRef]
- He, Z.; Dai, W.; Yu, J.; Pan, L.; Xiao, X.; Lu, S.; Jiang, N. Enhanced thermal and mechanical properties of polyimide composites by mixing thermotropic liquid crystalline epoxy grafted aluminum nitride. J. Polym. Res. 2014, 21, 595. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, X.; Wan, J.; Xiao, Y.; Fan, X. Preparation of AlN microspheres/UHMWPE composites for insulating thermal conductors. RSC Adv. 2016, 6, 80262–80267. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Harada, M.; Hamaura, N.; Ochi, M.; Agari, Y. Thermal conductivity of liquid crystalline epoxy/BN filler composites having ordered network structure. Compos. Part B Eng. 2013, 55, 306–313. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.; Yang, N.; Qin, L.; Grami, M.E.; Zhang, Q.; Wang, N.; Qu, X. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]
- Yang, N.; Xu, C.; Hou, J.; Yao, Y.; Zhang, Q.; Grami, M.E.; He, L.; Wanga, N.; Qu, X. Preparation and properties of thermally conductive polyimide/boron nitride composites. RSC Adv. 2016, 6, 18279–18287. [Google Scholar] [CrossRef]
- Wu, K.; Lei, C.; Yang, W.; Chai, S.; Chen, F.; Fu, Q. Surface modification of boron nitride by reduced graphene oxide for preparation of dielectric material with enhanced dielectric constant and well-suppressed dielectric loss. Compos. Sci. Technol. 2016, 134, 191–200. [Google Scholar] [CrossRef]
- Mittal, G.; Rhee, K.Y.; Park, S.J. Processing and characterization of PMMA/PI composites reinforced with surface functionalized hexagonal boron nitride. Appl. Surf. Sci. 2017, 415, 49–54. [Google Scholar] [CrossRef]
- Im, H.; Kim, J. The effect of Al2O3 doped multi-walled carbon nanotubes on the thermal conductivity of Al2O3/epoxy terminated poly(dimethylsiloxane) composites. Carbon 2011, 49, 3503–3511. [Google Scholar] [CrossRef]
- Hsu, S.; Wu, M.C.; Chen, S.; Chuang, C.M.; Lin, S.H.; Su, W.F. Synthesis, morphology and physical properties of multi-walled carbon nanotube/biphenyl liquid crystalline epoxy composites. Carbon 2012, 50, 896–905. [Google Scholar] [CrossRef]
- Ma, A.; Chen, W.; Hou, Y. Mechanical and Thermal Conductivities of MWCNTs/Si3N4/Epoxy Composites. Polym. Plast. Technol. Eng. 2013, 52, 1590–1594. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Ma, C.C.M.; Chiang, J.C.; Ho, K.K.; Chou, T.Y.; Xie, X.; Tsai, C.H.; Changa, L.H.; Hsieh, C.K. Thermally conductive and electrically insulating epoxy nanocomposites with thermally reduced graphene oxide–silica hybrid nanosheets. Nanoscale 2013, 13, 5863–5871. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Anwar, Z.; Muhammad, B. Perspectives of Epoxy/Graphene Oxide Composite: Significant Features and Technical Applications. Polym. Plast. Tech. Eng. 2016, 55, 704–722. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. [Google Scholar] [CrossRef]










| wt% of Si–BN | E’ a (MPa) | Tg b (°C) | CTE c (ppm/°C) | |||
|---|---|---|---|---|---|---|
| E’50 °C | E’150 °C | E’295 °C | Glassy Region | Rubbery Region | ||
| 0.00 | 2493 | 1968 | 124 | 228 | 70 | 157 |
| 0.50 | 3279 | 2464 | 157 | 249 | 63 | 143 |
| 1.00 | 3356 | 2577 | 166 | 256 | 60 | 136 |
| 2.00 | 3524 | 2795 | 189 | 267 | 56 | 122 |
| 5.00 | 3698 | 2812 | 193 | 271 | 53 | 117 |
| wt% of Si–BN | Td 5% a (°C) | Td 10% b (°C) | Char Yield (%) at 800 °C |
|---|---|---|---|
| 0.00 | 344 | 372 | 40.1 |
| 0.50 | 349 | 376 | 42.9 |
| 1.00 | 355 | 379 | 45.3 |
| 2.00 | 363 | 384 | 49.7 |
| 5.00 | 362 | 382 | 56.6 |
| 5.00 wt% of Si–BN | E’ (MPa) | Tg (°C) | CTE (ppm/°C) | Td 10% (°C) | Char Yield at 800 °C (%) | Thermal Conductivity (W/m·K) | |
|---|---|---|---|---|---|---|---|
| E’150 °C | Glassy Region | Rubbery Region | |||||
| K–LCE/BN | 2812 | 271 | 53 | 117 | 382 | 56.6 | 0.58 |
| DGEBA/BN | 2588 | 262 | 55 | 121 | 369 | 51.1 | 0.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-S.; Hsu, S.L.-C.; Ho, T.-H.; Jheng, L.-C.; Hsiao, Y.-H. Preparation and Thermomechanical Properties of Ketone Mesogenic Liquid Crystalline Epoxy Resin Composites with Functionalized Boron Nitride. Polymers 2020, 12, 1913. https://doi.org/10.3390/polym12091913
Lin Y-S, Hsu SL-C, Ho T-H, Jheng L-C, Hsiao Y-H. Preparation and Thermomechanical Properties of Ketone Mesogenic Liquid Crystalline Epoxy Resin Composites with Functionalized Boron Nitride. Polymers. 2020; 12(9):1913. https://doi.org/10.3390/polym12091913
Chicago/Turabian StyleLin, Yi-Sheng, Steve Lien-Chung Hsu, Tsung-Han Ho, Li-Cheng Jheng, and Yu-Hsiang Hsiao. 2020. "Preparation and Thermomechanical Properties of Ketone Mesogenic Liquid Crystalline Epoxy Resin Composites with Functionalized Boron Nitride" Polymers 12, no. 9: 1913. https://doi.org/10.3390/polym12091913