Micro/Nanostructured Coating for Cotton Textiles That Repel Oil, Water, and Chemical Warfare Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Si NPs Solution
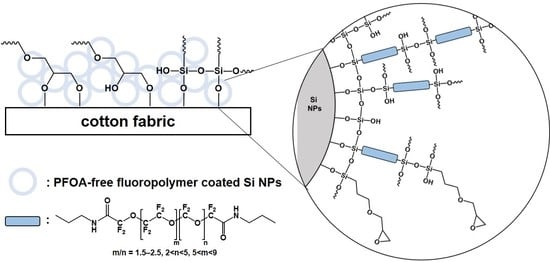
2.3. PFOA-Free Fluoropolymer-Coated Si NPs (OmniBlock)
2.4. Dip–Dry–Cure OmniBlock Coating
2.5. Characterization of Surfaces
2.6. Liquid Repellency Measurements
2.7. Mechanical Properties
3. Results and Discussion
3.1. OmniBlock-Coated Cotton Fabric
3.2. Liquid-Repellence Properties of OmniBlock-Coated Cotton Fabric
3.3. Mechanical Properties of OmniBlock-Coated Cotton Fabric
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parhizkar, M.; Zhao, Y.; Wunagi, X.; Lin, T. Photostability and durability properties of photochromic organosilica coating on fabric. J. Eng. Fibers Fabr. 2014, 9, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Berendjchi, A.; Khajavi, R.; Yazdanshenas, M.E. Fabrication of superhydrophobic and antibacterial surface on cotton fabric by doped silica-based sols with nanoparticles of copper. Nanoscale Res. Lett. 2011, 6, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Satyaprasad, A.; Jain, V.; Nema, S.K. Deposition of superhydrophobic nanostructured Teflon-like coating using expanding plasma arc. Appl. Surf. Sci. 2007, 253, 5462–5466. [Google Scholar] [CrossRef]
- Martines, E.; Seunarine, K.; Morgan, H.; Gadegaard, N.; Wilkinson, C.D.W.; Riehle, M.O. Superhydrophobicity and superhydrophilicity of regular nanopatterns. Nano Lett. 2005, 5, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, A.; Shevchenko, A.; Ovchinnikov, V.; Priimagi, A.; Kaivola, M. Optical interference lithography using azobenzene-functionalized polymers for micro- and nanopatterning of silicon. Adv. Mater. 2011, 23, 4174–4177. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Gao, S.; Koshizaki, N. Ordered Co3O4 hierarchical nanorod arrays: Tunable superhydrophilicity without UV irradiation and transition to superhydrophobicity. J. Mater. Chem. 2009, 19, 8366–8371. [Google Scholar] [CrossRef]
- Notsu, H.; Kubo, W.; Shitanda, I.; Tatsuma, T. Super-hydrophobic/super-hydrophilic patterning of gold surfaces by photocatalytic lithography. J. Mater. Chem. 2005, 15, 1523–1527. [Google Scholar] [CrossRef]
- Yang, X.; Tay, B.; Po, W.K.P. Fabrication of three-dimensional ZnO-Carbon nanotube (CNT) hybrids using self-assembled CNT micropatterns as framework. J. Phys. Chem. C 2007, 111, 17254–17259. [Google Scholar]
- Ofir, Y.; Samanta, B.; Arumugam, P.; Rotello, V.M. Controlled fluorination of FePt nanoparticles: Hydrophobic to superhydrophobic surfaces. Adv. Mater. 2007, 19, 4075–4079. [Google Scholar] [CrossRef]
- Wang, T.; Hu, W.; Dong, S. A general route to transform normal hydrophilic cloths into superhydrophobic surfaces. Chem. Commun. 2007, 18, 1849–1851. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, F.; Sun, J. A facile layer-by-layer deposition process for the fabrication of highly transparent superhydrophobic coatings. Chem. Commun. 2009, 2730–2732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, H.; Sun, J.; Shen, J. Layer-by-layer deposition of poly(diallyldimethylammonium chloride) and sodium silicate multilayers on silica-sphere-coated substrate-facile method to prepare a superhydrophobic surface. Chem. Mater. 2007, 19, 948–953. [Google Scholar] [CrossRef]
- Zhai, L.; Cebeci, F.C.; Cohen, R.C.; Rubner, M.R. Stable superhydrophobic coatings from polyelectrolyte multilayers. Nano Lett. 2004, 4, 1349–1353. [Google Scholar] [CrossRef]
- García, N.; Benito, E.; Guzmán, J.; Tiemblo, P. Use of p-toluenesulfonic acid for the controlled grafting of alkoxysilanes onto silanol containing surfaces: Preparation of tunable hydrophilic, hydrophobic, and super-hyerophobic silica. J. Am. Chem. Soc. 2007, 129, 5052–5069. [Google Scholar] [CrossRef]
- Sparks, B.J.; Hoff, E.F.; Xiong, L.; Goetz, J.T.; Patton, D.L. Superhydrophobic hybrid inorganic-organic thiol-ene surfaces fabricated via spray-deposition and photopolymerization. ACS Appl. Mater. Interfaces 2013, 5, 1811–1817. [Google Scholar] [CrossRef]
- Faustini, M.; Nicole, L.; Boissiere, C.; Innocenzi, P.; Sanchez, C.; Grosso, D. Hydrophobic, antireflective, self-cleaning, and antifogging sol-gel coatings: An example of multifunctional nanostructured materials for photovoltaic cells. Chem. Mater. 2010, 22, 4406–4413. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Sakai, M.; Fujishima, A. Development of sol–gel processed semi-transparent and self-cleaning superhydrophobic coatings. J. Mater. Chem. A 2014, 2, 5548–5553. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Azaman, S.H.; Ahmad, N.N.R.; Leo, C.P.; Lim, G.W.; Chan, D.J.C.; Yee, H.M. Superhydrophobic coating of silica with photoluminescence properties synthesized from rice husk ash. Prog. Org. Coat. 2017, 111, 29–37. [Google Scholar] [CrossRef]
- Meng, J.; Lin, S.; Xiong, X. Preparation of breathable and superhydrophobic coating film via spray coating in combination with vapor-induced phase separation. Prog. Org. Coat. 2017, 107, 29–36. [Google Scholar] [CrossRef]
- Ishizaki, T.; Masuda, Y.; Sakamoto, M. Corrosion resistance and durability of superhydrophobic surface formed on magnesium alloy coated with nanostructured cerium oxide film and fluoroalkylsilane molecules in corrosive NaCl aqueous solution. Langmuir 2011, 27, 4780–4788. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, M.; Zhai, J.; Wang, J.; Jiang, L. Bioinspired construction of mg-li alloys surfaces with stable superhydrophobicity and improved corrosion resistance. Appl. Phys. Lett. 2008, 92, 183103. [Google Scholar] [CrossRef]
- Bayer, I.S.; Steele, A.; Martorana, P.J.; Loth, E. Fabrication of superhydrophobic polyurethane/organoclay nano-structured composites from cyclomethicone-in-water emulsions. Appl. Surf. Sci. 2010, 257, 823–826. [Google Scholar] [CrossRef]
- Gupta, D.; Gulrajani, M.L. Self-cleaning finishes for textiles. In Functional Finishes for Textiles: Improving Comfort, Performance and Protection; Paul, R., Ed.; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Boban, M.; Golovin, K.; Tobelmann, B.; Gupte, O.; Mabry, J.M.; Tuteja, A. Smooth, all-solid, low-hysteresis, omniphobic surfaces with enhanced mechanical durability. ACS Appl. Mater. Interfaces 2018, 10, 11406–11413. [Google Scholar] [CrossRef] [PubMed]
- Milionis, A.; Bayer, I.S.; Loth, E. Recent advances in oil-repellent surfaces. Int. Mater. Rev. 2016, 61, 101–126. [Google Scholar] [CrossRef]
- Hensel, R.; Neinhuis, C.; Werner, C. The springtail cuticle as a blueprint for omniphobic surfaces. Chem. Soc. Rev. 2016, 45, 323–341. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Liu, W.; Yang, M.; He, S.; Xie, Y.; Wang, Z. Synthesis of superhydrophobic fluoro-containing silica sol coatings for cotton textile by one-step sol-gel process. J. Sol-Gel Sci. Technol. 2018, 87, 455–463. [Google Scholar] [CrossRef]
- Mahltig, B.; Audenaert, F.; Bottcher, H. Hydrophobic silica sol coatings on textiles-the influence of solvent and sol concentration. J. Sol-Gel Sci. Technol. 2005, 34, 103–109. [Google Scholar] [CrossRef]
- Yang, M.; Liu, W.; Jiang, C.; He, S.; Xie, Y.; Wang, Z. Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol-gel process. Carbohydr. Polym. 2018, 197, 75–82. [Google Scholar] [CrossRef]
- Onar, N.; Mete, G. Development of water-, oil-repellent and flame-retardant cotton fabrics by organic-inorganic hybrid materials. J. Text. Inst. 2016, 107, 1463–1477. [Google Scholar] [CrossRef]
- Bae, G.B.; Min, B.G.; Jeong, Y.G.; Lee, S.C.; Jang, J.H.; Koo, G.H. Superhydrophobicity of cotton fabrics treated with silica nano particles and water-repellent agent. J. Colloid Interface Sci. 2009, 337, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Przybylak, M.; Maciejewski, H.; Dutkiewicz, A.; Dabeck, A.; Nowichi, M. Fabrication of superhydrophobic cotton fabrics by a simple chemical modification. Cellulose 2016, 23, 2185–2197. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cai, Z. Effect of acid-catalyzed sol-gel silica coating on the properties of cotton fabric. J. Text. Inst. 2012, 103, 1099–1107. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Shi, Q. Preparation of transparent ultrahydrophobic silica film by sol-gel process. J. Coat. Technol. Res. 2011, 130, 3862–3868. [Google Scholar] [CrossRef]
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Ma, W.; Wu, H.; Higaki, Y.; Otsuka, H.; Takahara, A. A non-sticky superhydrophobic surface prepared by self-assembly of fluoroalkyl phosphonic acid on a hierarchically micro/nanostructured alumina gel film. Chem. Commun. 2012, 48, 6824–6826. [Google Scholar] [CrossRef]
- Wei, C.; Tang, Y.; Zhang, G.; Zhang, Q.; Zhan, X.; Chen, F. Facile fabrication of highly omniphobic and self-cleaning surfaces based on water mediated fluorinated nanosilica aggregation. RSC Adv. 2016, 6, 74340–74348. [Google Scholar] [CrossRef]
- Satoh, K.; Nakazumi, H.; Morita, M. Novel fluorinated inorganic-organic finishing materials for nylon carpeting. Text. Res. J. 2004, 74, 1079–1084. [Google Scholar] [CrossRef]
- Truong, Q.; Koene, B.; Domino, J. Omniphobic coatings for self-cleaning and enhanced chemical/biological (CB) agent protective clothing. NSTI-Nanotechnology 2013, 1, 663–666. [Google Scholar]
- United States Environmental Protection Agency. Per- and Polyfluoroalkyl Substances (PFASx) under TSCA; Docket ID: EPA-HQ-OPPT-2006-0621; United States Environmental Protection Agency: Washington, DC, USA, 2006. [Google Scholar]
- Gao, Y.; He, C.; Huang, Y.; Qing, F. Novel water and oil repellent POSS-based organic/inorganic nanomaterial: Preparation, characterization and application to cotton fabrics. Polymer 2010, 51, 5997–6004. [Google Scholar] [CrossRef]
- Teaf, C.M.; Garber, M.M.; Covert, D.J.; Tuovila, B.J. Perfluorooctanoic acid (PFOA): Environmental Sources, Chemistry, Toxicology, and Potential Risks. Soil Sediment Contam. Int. J. 2019, 28, 258–273. [Google Scholar] [CrossRef]
- Ghahfarokhi, F.S.; Khoddami, A.; Mazrouei-Sebdani, Z.; Rahmatinejad, J.; Mohammadi, H. A new technique to prepare a hydrophobic and thermal insulating polyester woven fabric using electro-spraying of nano-porous silica powder. Surf. Coat. Technol. 2019, 366, 97–105. [Google Scholar] [CrossRef]
- Ruan, M.; Zhan, Y.; Wu, Y.; Wang, X.; Li, W.; Chen, Y.; Wei, M.; Wang, X.; Deng, X. Preparation of PTFE/PDMS superhydrophobic coating and its anti-icing performance. RSC Adv. 2017, 7, 41339–41344. [Google Scholar] [CrossRef] [Green Version]
- Mohsin, M.; Sarwar, N.; Ahmad, S.; Rasheed, A.; Ahmad, F.; Afzal, A.; Zafar, S. Maleic acid crosslinking of C-6 fluorocarbon as oil and water repellent finish on cellulosic fabrics. J. Clean. Prod. 2016, 112, 3525–3530. [Google Scholar] [CrossRef]
- Truong, Q.T.; Pomerantz, N. Military applications: Development of superomniphobic coatings, textiles and surfaces. In Waterproof and Water Repellent Textiles and Clothing; The Textile Institute, Part Three Water Repellent Textiles in Practice: Performance, Testing and Applications 17; Woodhead Publishing, Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gorzkowska-Sobas, A.A. Chemical Warfare Agents and Their Interactions with Solid Surfaces; FFI-Rapport 2013/00574; Norwegian Defense Research Establishment (FFI): Kjeller, Norway, 2013. [Google Scholar]
- Hakonen, A.; Rindzevicius, T.; Schmidt, M.S.; Andersson, P.O.; Juhlin, L.; Svedendahl, M.; Boisen, A.; Käll, M. Detection of nerve gases using surface-enhanced Raman scattering substrates with high droplet adhesion. Nanoscale 2016, 8, 1305–1308. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, F.; Periolatto, M.; Gozzelino, G. Sol-gel process for surface modification of leather. In Recent Applications in Sol-Gel Synthesis; Usha, C., Ed.; InTech: London, UK, 2017; Chapter 14; pp. 283–299. [Google Scholar]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Ono, K. Spherical silica gels precipitated from acid catalyzed teos solutions. J. Non-Cryst. Solids 1990, 232, 383–388. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Lei, Q.; Wu, Y.; Li, W. Fabrication of superhydrophobic composite coating based on fluorosilicone resin and silica nanoparticles. R. Soc. Open Sci. 2018, 5, 180598–180612. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, J.; Seeger, S.; Reifler, F.A. Water shedding angle: A new technique to evaluate the water-repellent properties of superhydrophobic surface. Text. Res. J. 2009, 80, 1565–1570. [Google Scholar] [CrossRef]
- Tiab, D.; Donaldson, E.C. Wettability. In Petrophysics, 3rd ed.; Gulf Proffessional Publishing, Elsevier Inc.: Amsterdam, The Netherlands, 2012; Chapter 6. [Google Scholar]
- Rani, K.V.; Sarma, B.; Sarma, A. Plasma treatment on cotton fabrics to enhance the adhesion of reduced Graphene Oxide for electro-conductive properties. Diam. Relat. Mater. 2018, 84, 77–85. [Google Scholar] [CrossRef]
- Bhat, N.V.; Netravali, A.N.; Gore, A.V.; Sathianarayanan, M.P.; Arolkar, G.A.; Deshmukh, R.R. Surface modification of cotton fabrics using plasma technology. Text. Res. J. 2011, 81, 1014–1026. [Google Scholar] [CrossRef]
- Castelvetro, V.; Fatarella, E.; Corsi, L.; Giaiacopi, S.; Ciardelli, G. Graft polymerization of functional acrylic monomers onto cotton fibers activated by continuous Ar plasma. Plasma Process. Polym. 2006, 3, 48–57. [Google Scholar] [CrossRef]
- Montarsolo, A.; Periolatto, M.; Zerbola, M.; Mossotti, R.; Ferrero, F. Hydrophobic sol-gel finishing for textiles: Improvement by plasma pre-treatment. Text. Res. J. 2013, 83, 1190–1200. [Google Scholar] [CrossRef]
- Xiong, D.; Liu, G.; Duncan, J.S. Diblock-copolymer-coated water- and oil-repellent cotton fabrics. Langmuir 2012, 28, 6911–6918. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Yang, W.; Niu, H.; Wei, X.; Fu, S.; Liu, S.; Shao, H.; Lin, T. Durable superoleophobic-superhydrophilic fabrics with high anti-oil-fouling property. RSC Adv. 2018, 8, 26939–26947. [Google Scholar] [CrossRef] [Green Version]
- Xue, F.; Jia, D.; Li, Y.; Jing, X. Facile preparation of a mechanically robust superhydrophobic acrylic polyurethane coating. J. Mater. Chem. A 2015, 3, 13856–13863. [Google Scholar] [CrossRef]
- Hozumi, A.; Takai, O. Preparation of ultra water-repellent films by microwave plasma-enhanced CVD. Thin Solid Films 1997, 303, 222–225. [Google Scholar] [CrossRef]
- Latthe, S.S.; Imai, H.; Ganesan, V.; Rao, A.V. Superhydrophobic silica films by sol-gel co-precursor method. Appl. Surf. Sci. 2009, 256, 217–222. [Google Scholar] [CrossRef]
- Sarkar, D.K.; Brassard, D.; Khakani, M.A.E.; Ouellet, L. Dielectric properties of sol-gel derived high-k titanium silicate thin films. Thin Solid Films 2007, 515, 4788–4793. [Google Scholar] [CrossRef]
- Brassard, J.-D.; Sarkar, D.K.; Perron, J. Fluorin based superhydrophobic coatings. Appl. Sci. 2012, 2, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Facio, D.S.; Carrascosa, L.A.M.; Mosquera, M.J. Producing lasting amphiphobic building surfaces with self-cleaning properties. Nanotechnology 2017, 28, 265601. [Google Scholar] [CrossRef] [PubMed]
- Dirè, S.; Tagliazucca, V.; Callone, E.; Quaranta, A. Effect of functional groups on condensation and properties of sol-gel silica nanoparticles prepared by direct synthesis from organoalkoxysilanes. Mater. Chem. Phys. 2011, 126, 909–917. [Google Scholar] [CrossRef]
- Ceylan, Ö.; Landuyt, L.V.; Rahier, H.; Clerck, K.D. The effect of water immersion on the thermal degradation of cotton fibers. Cellulose 2013, 20, 1603–1612. [Google Scholar] [CrossRef]
- Protásio, T.P.; Guimarães, M.G., Jr.; Mirmehdi, S.; Fernando, P.F.; Napoli, A.; Knovack, K.M. Combustion of biomass and charcoal made from babassu nutshell. CERNE 2017, 23, 1–10. [Google Scholar]
- Bär, R.; Widmaier, S.; Levkin, P. Facile fabrication of robust superhydrophobic surfaces: Comparative investigation. RSC Adv. 2016, 6, 98257–98266. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, D.L.; Bardy, R.F., Jr.; Lam, K.; Schmidt, D.C.; Chaudhury, M.K. Contact angle hysteresis, adhesion, and marine biofouling. Langmuir 2004, 20, 2830–2836. [Google Scholar] [CrossRef]
- Byun, H.-R.; Ha, Y.-G. Non-wetting superhydrophobic surface enabled by one-step spray coating using molecular self-assembled nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 5515–5519. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Tao, R.; Zhu, Y.; Lv, C.; Zhu, Y. Fabrication of superhydrophobic fiber fabric/epoxy composites coating on aluminum substrate with long-lived wear resistance. RSC Adv. 2016, 6, 95556–95563. [Google Scholar] [CrossRef]
- Woodward, J.T.; Gwin, H.; Schwartz, D.K. Contact angles on surfaces with mesoscopic chemical heterogeneity. Langmuir 2000, 16, 2957–2961. [Google Scholar] [CrossRef]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, M.; Ueda, Y. The lowest surface free energy based on –CF3 alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagels, H.F.; Wu, D.; de With, G.; Ming, W. Biomimetic superhydrophobic and highly oleophobic cotton textiles. Langmuir 2007, 23, 13158–13163. [Google Scholar] [CrossRef] [PubMed]
- Shibuichi, S.; Onda, T.; Satoh, N.; Tsujii, K. Super-water-repellent fractal surfaces. Langmuir 1996, 12, 2125–2127. [Google Scholar]
- Erbil, H.Y.; Demirel, A.L.; Avci, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef]
- Yeerken, T.; Wang, G.; Li, H.; Liu, H.; Yu, W. Chemical stable, superhydrophobic and self-cleaning fabrics prepared by two-step coating of a polytetrafluoroethylene membrane and silica nanoparticles. Text. Res. J. 2019, 89, 4827–4841. [Google Scholar] [CrossRef]
- Jung, H.; Kim, M.; Jang, S. Liquid-repellent textile surfaces using zirconium (Zr)-based porous materials and a polyhedral oligomeric silsesquioxane coating. J. Colloid Interface Sci. 2020, 563, 363–369. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Hydrophobic and flame-retardant finishing of cotton fabrics for water-oil separation. Cellulose 2020, 27, 4145–4159. [Google Scholar] [CrossRef]
- Jeong, S.A.; Kang, T.J. Superhydrophobic and transparent surfaces on cotton fabrics coated with silica nanoparticles for hierarchical roughness. Text. Res. J. 2017, 8, 552–560. [Google Scholar] [CrossRef]
- Wang, H.; Ding, H.; Xue, Y.; Wang, X.; Lin, T. Superhydrophobic fabrics from hybrid silica sol-gel coatings: Structural effect of precursors on wettability and washing durability. J. Mater. Res. 2010, 25, 1336–1343. [Google Scholar] [CrossRef] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.; Jung, H.; Jung, H.; Lee, J. Micro/Nanostructured Coating for Cotton Textiles That Repel Oil, Water, and Chemical Warfare Agents. Polymers 2020, 12, 1826. https://doi.org/10.3390/polym12081826
Kwon J, Jung H, Jung H, Lee J. Micro/Nanostructured Coating for Cotton Textiles That Repel Oil, Water, and Chemical Warfare Agents. Polymers. 2020; 12(8):1826. https://doi.org/10.3390/polym12081826
Chicago/Turabian StyleKwon, Jihyun, Hyunsook Jung, Heesoo Jung, and Juno Lee. 2020. "Micro/Nanostructured Coating for Cotton Textiles That Repel Oil, Water, and Chemical Warfare Agents" Polymers 12, no. 8: 1826. https://doi.org/10.3390/polym12081826