Preparation and Barrier Performance of Layer-Modified Soil-Stripping/Cassava Starch Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Preparation
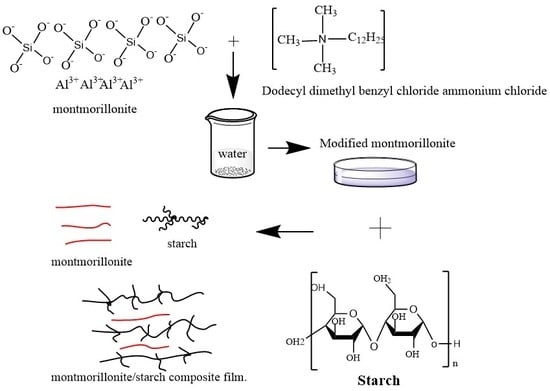
2.2.1. Preparation of Organic Montmorillonite
2.2.2. Preparation of Magnetized Organic Montmorillonite
2.2.3. Preparation of Cassava Starch/Modified Montmorillonite Composite Films
3. Testing and Characterization
3.1. Apparent Morphological Characterization
3.2. Barrier Performance
3.3. Mechanical Properties
3.4. Infrared Spectroscopy
3.5. X-ray Diffraction (XRD)
3.6. Thermal Stability
3.7. Contact Angle
4. Results
4.1. Apparent Morphological Analysis
4.2. Analysis of the Barrier Performance
4.3. Analysis of Mechanical Properties
4.4. Infrared Spectral Analysis
4.5. XRD Analysis
4.6. Thermal Stability Analysis
4.7. Surface Hydrophobicity Characterization
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Moraes, J.O.; Scheibe, A.S.; Sereno, A.; Laurindo, J.B. Scale-up of the production of cassava starch based films using tape-casting. J. Food Eng. 2013, 119, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.R. Preparation and Properties of High Water-ResistanceStarch Film. Master’s Thesis, Shandong Agricultural University, Shandong, China, 2014. [Google Scholar]
- Basiak, E.; Debeaufort, F.; Lenart, A. Effect of oil lamination between plasticized starch layers on film properties. Food Chem. 2016, 195, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Li, L.; Chen, H. Preparation, Characterization and adsorption properties of carboxymethyl cellulose/Fe3O4 composite nano-magnetic materials. Acta Chim. 2010, 68, 1461–1466. [Google Scholar]
- Li, C.J. Preparation of LDH/MMT Naocomposite based on the Exfoliation and Assembly Behavior of Montmorillonite. Master’s Thesis, Guilin University of Technology, Guilin, China, 2015. [Google Scholar]
- Savaş, L.A.; Savaş, S. Interrelation between contact angle and interlayer spacıng of montmorıllonıte clay used ın polymer anocomposıtes. Polym. Polym. Compos. 2013, 21, 129–132. [Google Scholar]
- Savas, L.A.; Hancer, M. Montmorillonite reinforced polymer nanocomposite antibacterial film. Appl. Clay Sci. 2015, 108, 40–44. [Google Scholar] [CrossRef]
- Dong, T.L.G.; Hu, H.; Liang, X.H. Preparation and application of polycaprolactone / montmorillonite / chitosan film. Packag. Eng. 2014, 35, 1–6. [Google Scholar]
- Liu, G.C. Nano-Montmorillonit modified PVOH-Based Composite Packaging Material and Its Effects on Salted Duck Eggs. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2014. [Google Scholar]
- Liu, S.Y. Effect of Starch-Based Nanocomposite Films Multi-Scale Structures on Water Vapor and Oxygen Barrier Property. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2018. [Google Scholar]
- Charifou, R.; Espuche, E.; Gouanvé, F.; Dubost, L.; Monaco, B. SiO x and SiO x C z H w mono- and multi-layer deposits for improved polymer oxygen and water vapor barrier properties. J. Membr. Sci. 2016, 500, 245–254. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Abdelsalam, S.I.; Vafai, K. Combined effects of magnetic field and rheological properties on the peristaltic flow of a compressible fluid in a microfluidic channel. Eur. J. Mech. B Fluid. 2017, 65, 398–411. [Google Scholar] [CrossRef]
- Abdelsalam, S.I.; Bhatti, M.M. The impact of impinging TiO2 nanoparticles in Prandtl nanofluid along with endoscopic and variable magnetic field effects on peristaltic blood flow. Multidiscip. Modeling Mater. Struct. 2018, 14, 530–548. [Google Scholar] [CrossRef]
- Mekheimer, K.S.; Komy, S.R.; Abdelsalam, S.I. Simultaneous effects of magnetic field and space porosity on compressible Maxwell fluid transport induced by a surface acoustic wave in a microchannel. Chin. Phys. B 2013, 22, 327–336. [Google Scholar]
- Zhou, J.M.; Tang, X.Z. Study on the structure and properties of montmorillonite composite film modified by starch-polyvinylalcohol-soyprotein. J. Chin. Cereals Oils Assoc. 2015, 30, 29–33. [Google Scholar]
- Fan, M.D.; Gou, H.J. Controllable Synthesis and characterization of Zero-valent Iron Nanopapticles directed by interfacial interactions of montmorillonite and polyvinylpyrrolidone. Chem. Ind. Eng. Prog. 2016, 35, 3563–3569. [Google Scholar]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Rheological Behavior of Reaction Mixtures during the Graft Copolymerization of Cassava Starch with Acrylic Acid; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; Volume 57. [Google Scholar]
- Al-Hassan, A.A.; Norziah, M.H. Starch-gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Shan, S.Y.; Jia, Q.M.; Jiang, L.H.; Wang, Y.M. Anticorrosive Properties of Epoxy/Polyurethane IPNs Coatings Modified by Organic Montmorillonite. In Materials Science Forum, Proceedings of the 7th International Forum on Advanced Material Science and Technology, Dalian, China, 26–28 June 2011; Trans Tech Publications: Stafa-Zurich, Switzerland, 2011; Volume 675–677, pp. 481–484. [Google Scholar]
- Ortega, F.; Giannuzzi, L.; Arce, V.B.; García, M.A. Active compose starch films containing green synthetized silver nanoparticles. Food Hydrocoll. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Yu, L.; Dexin, Y.; Tao, Y.; Ning, L.; Hao, D. The Stability of Intercalated Sericite by Cetyl Trimethylammonium Ion under Different Conditions and the Preparation of Sericite/Polymer Nanocomposites. Polymers 2019, 11, 900. [Google Scholar]
- Zhang, H.; Zhang, J.; Gao, Y.; Wang, W.; Dong, H.; Hou, H.; Liu, X. Effect of modification extent of montmorillonite on the performance of starch nanocomposite films. Starch-Starke 2017, 69, 1700088. [Google Scholar] [CrossRef]
- Abdelsalam, S.I.; Sohail, M. Numerical Approach of Variable Thermophysical Features of Dissipated Viscous Nanofluid Comprising Gyrotactic Micro-Organisms. Pramana 2020, 94, 67. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, Z.; Wu, S.; Tan, J.; Meng, K. Preparation and characterization of novel cationic-nonionic organo-montmorillonite. Mater. Express 2015, 5, 180–190. [Google Scholar] [CrossRef]
- Hu, X.; Ke, Y. The influence of organic modified montmorillonite on the solution properties of copolymer containing beta-cyclodextrin. J.Polym. Res. 2020, 27, 19. [Google Scholar] [CrossRef]
- Xi, Y.; Frost, R.L.; He, H. Modification of the surfaces of Wyoming montmorillonite by the cationic surfactants alkyl trimethyl, dialkyl dimethyl, and trialkyl methyl ammonium bromides. J. Colloid Interface Sci. 2006, 305, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Jai, H.; Li, S.X. Study on organic intercalation modified montmorillonite by mechanochemical method. China Petroleum Chem. Stand. Qual. 2012, 33, 26–27+35. [Google Scholar]
- Liu, Q.H.; Cheng, H.F.; Hao, R.W. Quaternary ammonium salt and alkylamine intercalated kaolinite structure and thermodynamic comparison. J. Chin. Ceram. Soc. 2019, 47, 90–97. [Google Scholar]
- Shang, T.H. Preparation of Magnetic Modified Graphene Oxide and Research about Barrier Properties of Epoxy with Aligned Graphene Oxide. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2017. [Google Scholar]
- Eldesoky, I.M.; Abdelsalam, S.I.; El-Askary, W.A.; Ahmed, M.M. Concurrent Development of Thermal Energy with Magnetic Field on a Particle-Fluid Suspension through a Porous Conduit; Springer, U.S. Ltd.: New York, NY, USA, 2019; p. 9. [Google Scholar]














| Sample | 2θ (°) | Interlayer Spacing (nm) |
|---|---|---|
| MMT | 5.28 | 0.16 |
| 0-1 DTAC | 5.42 | 0.18 |
| 1-1 DTAC | 5.54 | 0.21 |
| 2-1 DTAC | 6.18 | 1.49 |
| 1-2 DTAC | 6.12 | 0.95 |
| 1-3 DTAC | 6.13 | 1.01 |
| 1-2 DDBAC | 5.88 | 0.38 |
| 1-2STAC | 6.16 | 1.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Han, X.; Chen, H.; An, S.; Zhao, H.; Xu, H.; Huang, C.; Wang, S.; Liu, Y. Preparation and Barrier Performance of Layer-Modified Soil-Stripping/Cassava Starch Composite Films. Polymers 2020, 12, 1611. https://doi.org/10.3390/polym12071611
Huang L, Han X, Chen H, An S, Zhao H, Xu H, Huang C, Wang S, Liu Y. Preparation and Barrier Performance of Layer-Modified Soil-Stripping/Cassava Starch Composite Films. Polymers. 2020; 12(7):1611. https://doi.org/10.3390/polym12071611
Chicago/Turabian StyleHuang, Lijie, Xiaoxue Han, Haobin Chen, Shuxiang An, Hanyu Zhao, Hao Xu, Chongxing Huang, Shuangfei Wang, and Yang Liu. 2020. "Preparation and Barrier Performance of Layer-Modified Soil-Stripping/Cassava Starch Composite Films" Polymers 12, no. 7: 1611. https://doi.org/10.3390/polym12071611