Polymer-Based Scaffolds for Soft-Tissue Engineering
Abstract
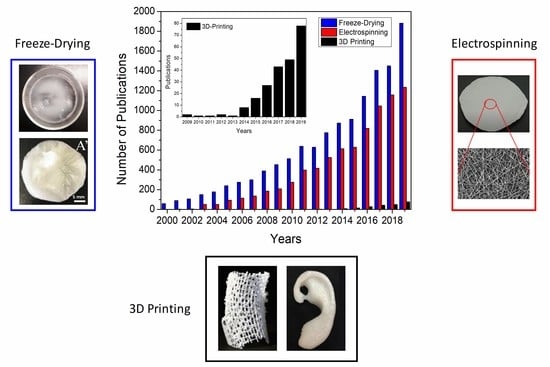
:1. Introduction
2. Freeze-Drying
2.1. Technique
2.2. Current Trends
3. Electrospinning
3.1. Technique
3.2. Current Trends
4. 3D Printing
4.1. Technique
4.1.1. Stereolithography (SLA) 3D Printing
4.1.2. Fused Deposition Modeling (FDM)
4.1.3. Selective Laser Sintering (SLS)
4.1.4. Material Jetting (MJ) and Bending Jetting (BJ) 3D Printing
4.2. Current Trends
5. Comparison and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dean, M.N.; Swanson, B.O.; Summers, A.P. Biomaterials: Properties, variation and evolution. Integr. Comp. Biol. 2009, 49, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier Science: Washington, DC, USA, 2012; ISBN 9780080877808. [Google Scholar]
- Williams, D.F. Definitions in Biomaterials: Proceedings of a Consensus Conference of the European Society for Biomaterials, Chester, England, March 3–5, 1986; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1987. [Google Scholar]
- Hench, L.L. Biomaterials: A forecast for the future. Biomaterials 1998, 19, 1419–1423. [Google Scholar] [CrossRef]
- Nations, U. United Nations Demographic Yearbook 2018; Demographic Yearbook; UN: New York, NY, USA, 2020; ISBN 9789211483208. [Google Scholar]
- Nicholson, J.W.; Connor, J.A. Synthetic materials in medicine. In The Chemistry of Medical and Dental Materials; Nicholson, J.W., Connor, J.A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2002; pp. 1–24. [Google Scholar]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.-T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- dos Santos, V.; Brandalise, R.N.; Savaris, M. Engineering of Biomaterials; Topics in Mining, Metallurgy and Materials Engineering; Springer International Publishing: Cham, Germany, 2017; ISBN 978-3-319-58606-9. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Koh, C.J.; Atala, A. Tissue Engineering, Stem Cells, and Cloning: Current Concepts and Future Trends. In BT—Regenerative and Cell Therapy; Keating, A., Dicke, K., Gorin, N., Weber, R., Graf, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 35–67. [Google Scholar]
- Meyer, U.; Meyer, T.; Handschel, J.; Wiesmann, H.P. Fundamentals of Tissue Engineering and Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-77754-0. [Google Scholar]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. S4), 467–479. [Google Scholar] [CrossRef] [Green Version]
- Tariverdian, T.; Sefat, F.; Gelinsky, M.; Mozafari, M. 10—Scaffold for bone tissue engineering. In Woodhead Publishing Series in Biomaterials; Mozafari, M., Sefat, F., Atala, A.B.T.-H., Eds.; Woodhead Publishing: Melbourne, Australia, 2019; pp. 189–209. ISBN 978-0-08-102563-5. [Google Scholar]
- Sultana, N. Biodegradable Polymer-Based Scaffolds for Bone Tissue Engineering; SpringerBriefs in Applied Sciences and Technology; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642348020. [Google Scholar]
- Vallet-Regi, M.; Munuera, L. Biomateriales: Aquí y Ahora; Editorial Dykinson: Madrid, Spain, 2000. [Google Scholar]
- Burdick, J.A.; Mauck, R.L. Biomaterials for Tissue Engineering Applications: A Review of the Past and Future Trends; Springer: Vienna, Austria, 2010; ISBN 9783709103852. [Google Scholar]
- Okamoto, M. 2—The role of scaffolds in tissue engineering. In Woodhead Publishing Series in Biomaterials; Mozafari, M., Sefat, F., Atala, A.B.T.-H., Eds.; Woodhead Publishing: Melbourne, Australia, 2019; pp. 23–49. ISBN 978-0-08-102563-5. [Google Scholar]
- Sachlos, E.; Czemuszka, J.T. Making tissue engineering scaffolds work. Review on the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cells Mater. 2003, 5, 29–40. [Google Scholar] [CrossRef]
- Sionkowska, A. Biopolymeric nanocomposites for potential biomedical applications. Polym. Int. 2016, 65, 1123–1131. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Seal, B.L.; Otero, T.C.; Panitch, A. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Stratton, S.; Manoukian, O.S.; Patel, R.; Wentworth, A.; Rudraiah, S.; Kumbar, S.G. Polymeric 3D printed structures for soft-tissue engineering. J. Appl. Polym. Sci. 2018, 135, 45569. [Google Scholar] [CrossRef] [Green Version]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Sant, S.; Hwang, C.M.; Lee, S.-H.; Khademhosseini, A. Hybrid PGS-PCL microfibrous scaffolds with improved mechanical and biological properties. J. Tissue Eng. Regen. Med. 2011, 5, 283–291. [Google Scholar] [CrossRef]
- Gümüşderelioğlu, M.; Dalkıranoğlu, S.; Aydın, R.S.T.; Çakmak, S. A novel dermal substitute based on biofunctionalized electrospun PCL nanofibrous matrix. J. Biomed. Mater. Res. Part A 2011, 98A, 461–472. [Google Scholar] [CrossRef]
- Naghieh, S.; Foroozmehr, E.; Badrossamay, M.; Kharaziha, M. Combinational processing of 3D printing and electrospinning of hierarchical poly(lactic acid)/gelatin-forsterite scaffolds as a biocomposite: Mechanical and biological assessment. Mater. Des. 2017, 133, 128–135. [Google Scholar] [CrossRef]
- Ma, P.X.; Langer, R. Degradation, Structure and Properties of Fibrous Nonwoven Poly(Glycolic Acid) Scaffolds for Tissue Engineering. MRS Proc. 1995, 394, 99. [Google Scholar] [CrossRef]
- Celikkin, N.; Rinoldi, C.; Costantini, M.; Trombetta, M.; Rainer, A.; Święszkowski, W. Naturally derived proteins and glycosaminoglycan scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2017, 78, 1277–1299. [Google Scholar] [CrossRef] [PubMed]
- Hochleitner, G.; Jungst, T.; Brown, T.D.; Hahn, K.; Moseke, C.; Jakob, F.; Dalton, P.D.; Groll, J. Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing. Biofabrication 2015, 7, 35002. [Google Scholar] [CrossRef]
- Thomson, R.C.; Wake, M.C.; Yaszemski, M.J.; Mikos, A.G. Biodegradable polymer scaffolds to regenerate organs. In Biopolymers II; Peppas, N.A., Langer, R.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 245–274. [Google Scholar]
- Mikos, A.G.; Sarakinos, G.; Leite, S.M.; Vacanti, J.P.; Langer, R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials 1993, 14, 323–330. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C 2017, 78, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003, 21, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Fereshteh, Z. Freeze-drying technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Deng, Y., Kuiper, J., Eds.; Woodhead Publishing: Melburne, Australia, 2017; pp. 151–174. ISBN 9780081009802. [Google Scholar]
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Ghorbani, F.; Li, D.; Ni, S.; Zhou, Y.; Yu, B. 3D printing of acellular scaffolds for bone defect regeneration: A review. Mater. Today Commun. 2020, 22. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Khan, R.H.; Suman, R. 3D printing applications in bone tissue engineering. J. Clin. Orthop. Trauma 2020, 11, S118–S124. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Development of PVA/gelatin nanofibrous scaffolds for Tissue Engineering via electrospinning. Mater. Res. Express 2018, 5, 035401. [Google Scholar] [CrossRef]
- Maimouni, I.; Cejas, C.M.; Cossy, J.; Tabeling, P.; Russo, M. Microfluidics Mediated Production of Foams for Biomedical Applications. Micromachines 2020, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- de la Portilla, F.; Pereira, S.; Molero, M.; De Marco, F.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Microstructural, mechanical, and histological evaluation of modified alginate-based scaffolds. J. Biomed. Mater. Res. -Part A 2016, 104, 3107–3114. [Google Scholar] [CrossRef]
- Teimouri, A.; Azadi, M. Preparation and characterization of novel chitosan/nanodiopside/nanohydroxyapatite composite scaffolds for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 917–927. [Google Scholar] [CrossRef]
- de Groot, J.H.; Nijenhuis, A.J.; Bruin, P.; Pennings, A.J.; Veth, R.P.H.; Klompmaker, J.; Jansen, H.W.B. Use of porous biodegradable polymer implants in meniscus reconstruction. 1) Preparation of porous biodegradable polyurethanes for the reconstruction of meniscus lesions. Colloid Polym. Sci. 1990, 268, 1073–1081. [Google Scholar] [CrossRef]
- Whang, K.; Thomas, C.H.; Healy, K.E.; Nuber, G. A novel method to fabricate bioabsorbable scaffolds. Polymer (Guildf.) 1995, 36, 837–842. [Google Scholar] [CrossRef]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Healthc. Mater. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Viera Rey, D.F.; St-Pierre, J.-P. Fabrication techniques of tissue engineering scaffolds. In Handbook of Tissue Engineering Scaffolds: Volume One; Elsevier: Tehran, Iran, 2019; pp. 109–125. [Google Scholar]
- Tanasa, E.; Zaharia, C.; Hudita, A.; Radu, I.-C.; Costache, M.; Galateanu, B. Impact of the magnetic field on 3T3-E1 preosteoblasts inside SMART silk fibroin-based scaffolds decorated with magnetic nanoparticles. Mater. Sci. Eng. C 2020, 110, 110714. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; He, C.; Peng, S.; Gao, C.; Yang, Y.; Qi, F.; Feng, P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2020, 185, 108275. [Google Scholar] [CrossRef]
- Aradmehr, A.; Javanbakht, V. A novel biofilm based on lignocellulosic compounds and chitosan modified with silver nanoparticles with multifunctional properties: Synthesis and characterization. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 124952. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Akrami-Hasan-Kohal, M.; Ghorbani, M. An injectable chitosan-based hydrogel scaffold containing gold nanoparticles for tissue engineering applications. Int. J. Biol. Macromol. 2020, 154, 198–205. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Felix, M.; Romero, A.; Guerrero, A. Influence of the processing variables on the microstructure and properties of gelatin-based scaffolds by freeze-drying. J. Appl. Polym. Sci. 2019, 136, 1–8. [Google Scholar] [CrossRef]
- Zamanian, A.; Ghorbani, F.; Nojehdehian, H. Morphological comparison of PLGA/gelatin scaffolds produced by freeze casting and freeze drying methods. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014; Volume 467, pp. 108–111. [Google Scholar]
- Reys, L.L.; Silva, S.S.; Pirraco, R.P.; Marques, A.P.; Mano, J.F.; Silva, T.H.; Reis, R.L. Influence of freezing temperature and deacetylation degree on the performance of freeze-dried chitosan sca ff olds towards cartilage tissue engineering. Eur. Polym. J. 2017, 95, 232–240. [Google Scholar] [CrossRef]
- Schwarzenbach, M.S.; Reimann, P.; Thommen, V.; Hegner, M.; Mumenthaler, M.; Schwob, J.; Güntherodt, H.-J. Interferon α-2a interactions on glass vial surfaces measured by atomic force microscopy. PDA J. Pharm. Sci. Technol. 2002, 56, 78–89. [Google Scholar] [PubMed]
- Tucker, N.; Stanger, J.J.; Staiger, M.P.; Razzaq, H.; Hofman, K. The History of the Science and Technology of Electrospinning from 1600 to 1995. J. Eng. Fiber. Fabr. 2012, 7, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.I. Disintegration of water drops in an electric field. Proc. R. Soc. Lond. A 1964, 280, 383–398. [Google Scholar]
- Long, Y.Z.; Yan, X.; Wang, X.X.; Zhang, J.; Yu, M. Electrospinning; Elsevier Inc.: New York, NY, USA, 2018; ISBN 9780323512701. [Google Scholar]
- Stepanyan, R.; Subbotin, A.V.; Cuperus, L.; Boonen, P.; Dorschu, M.; Oosterlinck, F.; Bulters, M.J.H. Nanofiber diameter in electrospinning of polymer solutions: Model and experiment. Polymer (Guildf.) 2016, 97, 428–439. [Google Scholar] [CrossRef]
- Gugulothu, D.; Barhoum, A.; Nerella, R.; Ajmer, R.; Bechelany, M. Fabrication of Nanofibers: Electrospinning and Non-electrospinning Techniques. In Handbook of Nanofibers; Springer International Publishing: Cham, Germany, 2019; pp. 45–77. [Google Scholar]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [Green Version]
- Ghalia, M.A.; Dahman, Y. Advanced nanobiomaterials in tissue engineering. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: New York, NY, USA, 2016; pp. 141–172. [Google Scholar]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Jain, R.; Shetty, S.; Yadav, K.S. Unfolding the electrospinning potential of biopolymers for preparation of nanofibers. J. Drug Deliv. Sci. Technol. 2020, 57, 101604. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers (Basel) 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Almetwally, A.A.; El-Sakhawy, M.; Elshakankery, M.H.; Kasem, M.H. Technology of nano-fibers: Production techniques and properties-Critical review. J. Text. Assoc. 2017, 78, 5–14. [Google Scholar]
- Sukigara, S.; Gandhi, M.; Ayutsede, J.; Micklus, M.; Ko, F. Regeneration of Bombyx mori silk by electrospinning—part 1: Processing parameters and geometric properties. Polymer (Guildf.) 2003, 44, 5721–5727. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Effect of viscosity and electrical conductivity on the morphology and fiber diameter in melt electrospinning of polypropylene. Text. Res. J. 2013, 83, 606–617. [Google Scholar] [CrossRef]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of linear homopolymers of poly(methyl methacrylate): Exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer (Guildf.) 2005, 46, 4799–4810. [Google Scholar] [CrossRef]
- Eda, G.; Shivkumar, S. Bead-to-fiber transition in electrospun polystyrene. J. Appl. Polym. Sci. 2007, 106, 475–487. [Google Scholar] [CrossRef]
- Kim, B.; Park, H.; Lee, S.-H.; Sigmund, W.M. Poly(acrylic acid) nanofibers by electrospinning. Mater. Lett. 2005, 59, 829–832. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Moghe, A.K.; Hufenus, R.; Hudson, S.M.; Gupta, B.S. Effect of the addition of a fugitive salt on electrospinnability of poly(ɛ-caprolactone). Polymer (Guildf.) 2009, 50, 3311–3318. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Hong, Y.; Zhao, Y.; Qiu, S.; Wang, C.; Wei, Y. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Veleirinho, B.; Rei, M.F.; Lopes-DA-Silva, J.A. Solvent and concentration effects on the properties of electrospun poly(ethylene terephthalate) nanofiber mats. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 460–471. [Google Scholar] [CrossRef]
- Jarusuwannapoom, T.; Hongrojjanawiwat, W.; Jitjaicham, S.; Wannatong, L.; Nithitanakul, M.; Pattamaprom, C.; Koombhongse, P.; Rangkupan, R.; Supaphol, P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 2005, 41, 409–421. [Google Scholar] [CrossRef]
- Liu, J.; Kumar, S. Microscopic polymer cups by electrospinning. Polymer (Guildf.) 2005, 46, 3211–3214. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, Y.; Dong, C.; Sheng, J. Morphology of ultrafine polysulfone fibers prepared by electrospinning. Polym. Int. 2004, 53, 1704–1710. [Google Scholar] [CrossRef]
- Chowdhury, M.; Stylios, G. Effect of experimental parameters on morphology of electrospun Nylon 6 fibers. Int. J. Basic Appl. Sci. 2010, 10, 10–18. [Google Scholar]
- Yarin, A.L.; Pourdeyhimi, B.; Ramakrishna, S. Fundamentals and Applications of Micro and Nanofibers; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781107446830. [Google Scholar]
- Ko, F.K.; Wan, Y. Introduction to Nanofiber Materials; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781139021333. [Google Scholar]
- Amiraliyan, N.; Nouri, M.; Kish, M.H. Effects of some electrospinning parameters on morphology of natural silk-based nanofibers. J. Appl. Polym. Sci. 2009, 113, 226–234. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Lele, A.K.; Jog, J.P. Electrospinning of Nylon11: Effect of processing parameters on morphology and microstructure. Mater. Today Commun. 2015, 3, 141–148. [Google Scholar] [CrossRef]
- Theron, A.; Zussman, E.; Yarin, A.L. Electrostatic field-assisted alignment of electrospun nanofibres. Nanotechnology 2001, 12, 384–390. [Google Scholar] [CrossRef]
- Becker, A.; Zernetsch, H.; Mueller, M.; Glasmacher, B. A novel coaxial nozzle for in-process adjustment of electrospun scaffolds’ fiber diameter. Curr. Dir. Biomed. Eng. 2015, 1, 104–107. [Google Scholar] [CrossRef]
- Oğulata, R.T.; İçoğlu, H.İ. Interaction between effects of ambient parameters and those of other important parameters on electrospinning of PEI/NMP solution. J. Text. Inst. 2015, 106, 57–66. [Google Scholar] [CrossRef]
- Yuya, N.; Kai, W.; Kim, B.S.; Kim, I.S. Morphology controlled electrospun poly(vinyl pyrrolidone) fibers: Effects of organic solvent and relative humidity. J. Mater. Sci. Eng. 2010, 2, 97. [Google Scholar]
- Cho, D.; Zhmayev, E.; Joo, Y.L. Structural studies of electrospun nylon 6 fibers from solution and melt. Polymer (Guildf.) 2011, 52, 4600–4609. [Google Scholar] [CrossRef]
- Fashandi, H.; Karimi, M. Pore formation in polystyrene fiber by superimposing temperature and relative humidity of electrospinning atmosphere. Polymer (Guildf.) 2012, 53, 5832–5849. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core–Shell Fibers: Design, Roles, and Controllable Release Strategies in Tissue Engineering and Drug Delivery. Polymers (Basel) 2019, 11, 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Manjumeena, R.; Elakkiya, T.; Duraibabu, D.; Feroze Ahamed, A.; Kalaichelvan, P.T.; Venkatesan, R. “Green” biocompatible organic-inorganic hybrid electrospun nanofibers for potential biomedical applications. J. Biomater. Appl. 2015, 29, 1039–1055. [Google Scholar] [CrossRef]
- Rajzer, I.; Kurowska, A.; Jabłoński, A.; Jatteau, S.; Śliwka, M.; Ziąbka, M.; Menaszek, E. Layered gelatin/PLLA scaffolds fabricated by electrospinning and 3D printing- for nasal cartilages and subchondral bone reconstruction. Mater. Des. 2018, 155, 297–306. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Li, Y.; Jia, L.; Mo, X.; Jiang, G.; Zhou, G. 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem. Eng. J. 2020, 382, 122986. [Google Scholar] [CrossRef]
- Yoshikawa, M. Molecularly imprinted polymeric membranes. Bioseparation 2001, 10, 277–286. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Milosevic, B.; Frenot, A.; Ye, L. Generation of molecular recognition sites in electrospun polymer nanofibers via molecular imprinting. Macromolecules 2006, 39, 357–361. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tanioka, A.; Matsumoto, H. Molecularly imprinted nanofiber membranes. Curr. Opin. Chem. Eng. 2011, 1, 18–26. [Google Scholar] [CrossRef]
- Ghorani, B.; Tucker, N.; Yoshikawa, M. Approaches for the assembly of molecularly imprinted electrospun nanofibre membranes and consequent use in selected target recognition. Food Res. Int. 2015, 78, 448–464. [Google Scholar] [CrossRef]
- Su, A.; Al’Aref, S.J. Chapter 1—History of 3D Printing. In 3D Printing Applications in Cardiovascular Medicine; Al’Aref, S.J., Mosadegh, B., Dunham, S., Min, J.K.B.T., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 1–10. ISBN 978-0-12-803917-5. [Google Scholar]
- Li, T.; Chang, J.; Zhu, Y.; Wu, C. 3D Printing of Bioinspired Biomaterials for Tissue Regeneration. Adv. Healthc. Mater. 2020, 2000208. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Yao, H.; Wang, J.; Mi, S. Photo Processing for Biomedical Hydrogels Design and Functionality: A Review. Polymers 2017, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Mazzanti, V.; Malagutti, L.; Mollica, F. FDM 3D Printing of Polymers Containing Natural Fillers: A Review of their Mechanical Properties. Polymers 2019, 11, 1094. [Google Scholar] [CrossRef] [Green Version]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Serien, D.; Sugioka, K. Three-Dimensional Printing of Pure Proteinaceous Microstructures by Femtosecond Laser Multiphoton Cross-Linking. ACS Biomater. Sci. Eng. 2020, 6, 1279–1287. [Google Scholar] [CrossRef]
- Ng, W.L.; Chua, C.K.; Shen, Y.-F. Print Me An Organ! Why We Are Not There Yet. Prog. Polym. Sci. 2019, 97, 101145. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [Green Version]
- Rathan, S.; Dejob, L.; Schipani, R.; Haffner, B.; Möbius, M.E.; Kelly, D.J. Fiber Reinforced Cartilage ECM Functionalized Bioinks for Functional Cartilage Tissue Engineering. Adv. Healthc. Mater. 2019, 8, 1801501. [Google Scholar] [CrossRef] [PubMed]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, 1800672. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P.; et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kérourédan, O.; Rémy, M.; Oliveira, H.; Guillemot, F.; Devillard, R. Laser-Assisted Bioprinting of Cells for Tissue Engineering. Laser Print. Funct. Mater. 2018, 15, 349–373. [Google Scholar] [CrossRef]
- Ng, W.L.; Lee, J.M.; Yeong, W.Y.; Win Naing, M. Microvalve-based bioprinting-process, bio-inks and applications. Biomater. Sci. 2017, 5, 632–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.-W.; Lee, K.-X.A.; Yeong, W.Y.; Shen, Y.-F. Vat polymerization-based bioprinting—process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 22001. [Google Scholar] [CrossRef]
- Shie, M.-Y.; Shen, Y.-F.; Astuti, S.D.; Lee, A.K.-X.; Lin, S.-H.; Dwijaksara, N.L.B.; Chen, Y.-W. Review of Polymeric Materials in 4D Printing Biomedical Applications. Polymers 2019, 11, 1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Selleri, S.; Tonelli, A.; Pasquali, F.; Candiani, A.; Cucinotta, A.; Biasion, F.; Barozzi, M. Boosting accessibility of diagnostics tools for 3D printing, consumer electronics, digital imaging and open source software conversion (Conference Presentation). In Proceedings of the Optical Methods for Inspection, Characterization, and Imaging of Biomaterials IV, Munich, Germany, 24–26 June 2019; Ferraro, P., Ritsch-Marte, M., Grilli, S., Hitzenberger, C.K., Eds.; SPIE: Bellingham, WA, USA, 2019; p. 22, ISBN 9781510627994. [Google Scholar]
- Ng, W.L.; Chan, A.; Ong, Y.S.; Chua, C.K. Deep learning for fabrication and maturation of 3D bioprinted tissues and organs. Virtual Phys. Prototyp. 2020, 15, 340–358. [Google Scholar] [CrossRef]
- Kopp, A.; Smeets, R.; Gosau, M.; Kröger, N.; Fuest, S.; Köpf, M.; Kruse, M.; Krieger, J.; Rutkowski, R.; Henningsen, A.; et al. Effect of process parameters on additive-free electrospinning of regenerated silk fibroin nonwovens. Bioact. Mater. 2020, 5, 241–252. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef]
- Creff, J.; Courson, R.; Mangeat, T.; Foncy, J.; Souleille, S.; Thibault, C.; Besson, A.; Malaquin, L. Fabrication of 3D scaffolds reproducing intestinal epithelium topography by high-resolution 3D stereolithography. Biomaterials 2019, 221. [Google Scholar] [CrossRef]
- Dhinakaran, V.; Manoj Kumar, K.P.; Bupathi Ram, P.M.; Ravichandran, M.; Vinayagamoorthy, M. A review on recent advancements in fused deposition modeling. Mater. Today Proc. 2020, 27, 752–756. [Google Scholar] [CrossRef]
- Pu, N.A.S.M.; Haq, R.H.A.; Noh, H.M.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Materials Today: Proceedings Review on the fabrication of fused deposition modelling (FDM) composite filament for biomedical applications. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Yuan, S.; Strobbe, D.; Li, X.; Kruth, J.P.; Van Puyvelde, P.; Van der Bruggen, B. 3D printed chemically and mechanically robust membrane by selective laser sintering for separation of oil/water and immiscible organic mixtures. Chem. Eng. J. 2020, 385, 123816. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, and challenges. Prog. Mater. Sci. 2020, 100707. [Google Scholar] [CrossRef]
- Ziaee, M.; Crane, N.B. Binder jetting: A review of process, materials, and methods. Addit. Manuf. 2019, 28, 781–801. [Google Scholar] [CrossRef]
- Serpooshan, V.; Guvendiren, M. Editorial for the Special Issue on 3D Printing for Tissue Engineering and Regenerative Medicine. Micromachines 2020, 11, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, V.; Arruebo, M. Microfluidic production of inorganic nanomaterials for biomedical applications. In Microfluidics for Pharmaceutical Applications; Elsevier: Turku, Finland, 2019; pp. 179–216. ISBN 9780128126608. [Google Scholar]
- Jiang, X. Microfluidic Devices with Coarse Capillaries to Fabricate Bioengineering Products: Bubbles, Scaffolds and Nanoparticles; University College London: London, UK, 2019. [Google Scholar]
- Russo, M.; Bevilacqua, P.; Netti, P.A.; Torino, E. A Microfluidic Platform to design crosslinked Hyaluronic Acid Nanoparticles (cHANPs) for enhanced MRI. Sci. Rep. 2016, 6, 37906. [Google Scholar] [CrossRef]
- da Silva Morais, A.; Vieira, S.; Zhao, X.; Mao, Z.; Gao, C.; Oliveira, J.M.; Reis, R.L. Advanced Biomaterials and Processing Methods for Liver Regeneration: State-of-the-Art and Future Trends. Adv. Healthc. Mater. 2020, 9, 1901435. [Google Scholar] [CrossRef]
- Dalton, E.; Chai, Q.; Shaw, M.W.; McKenzie, T.J.; Mullins, E.S.; Ayres, N. Hydrogel-coated polyurethane/urea shape memory polymer foams. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1389–1395. [Google Scholar] [CrossRef]






| Parameters | General Effects on the Morphology of Fiber Mats | References | |
|---|---|---|---|
| Composition parameters | Concentration | Fiber diameter increases with the polymer concentration | [68,69] |
| A decrease in concentration leads to formation of beads | |||
| Viscosity | There is direct relationship with concentration that leads to similar effects | [70,71] | |
| An increase in viscosity may prevent a stable flow through the nozzle | |||
| Molecular weight of polymer | Similar effect than viscosity that grows with increasing molecular weight | [72,73] | |
| An increase may prevent the occurrence of beads | |||
| Conductivity | An increase favors the formation of uniform fibers free of beads | [74,75,76] | |
| It also favors a reduction in size (with some exceptions) | |||
| Surface tension | There is not a general trend between surface tension and fiber morphology | [70] | |
| Volatility | A low volatility level may impair solvent removal | [77,78] | |
| A high volatility level may lead to ribbon-like and porous fibers | |||
| Dielectric constant of solvent | High values of the solvent dielectric constant favor electrospinning | [79,80] | |
| A secondary solvent may be added to increase the dielectric constant | |||
| Processing parameters | Flow rate | Low flow rates give rise to small fiber diameters | [81,82] |
| High flow rates may prevent full solvent removal before the target | |||
| Voltage | There is no clear correlation between voltage and diameter of fibers | [75,83,84] | |
| High voltage values may lead to formation of beads along the fibers | |||
| Very high voltages may lead to formation of ultrathin secondary filaments | |||
| Nozzle–collector distance | A minimum distance is required to produce solvent-free fibers | [85,86] | |
| Too long or too short distances may lead to formation of beads | |||
| Type of nozzle | Coaxial nozzles may be used to produce hollow fibers | [41,87] | |
| Multiple nozzles are used to increase the production scale of fiber mats | |||
| Collector | Metallic collectors lead to smooth fibers and porous collectors to porous fibers | [41,88] | |
| Rotatory drum collectors may be used to control fiber alignment in the mat | |||
| Environmental | Temperature | A rise in temperature reduces the viscosity with its corresponding effects | [69,85,89] |
| Tend to reduce fiber size and may lead to bead formation at high concentration | |||
| It may also extend the polymer concentration window for electrospinning | |||
| Relative humidity (RH) | Low RH values anticipate evaporation and solidification, increasing fiber size | [90,91,92] | |
| High RH causes water condensation on the filaments and polymer precipitates. This effect leads to thick and porous fibers, even preventing their formation. | |||
| Conventional Technologies | Advantages | Drawbacks | References |
| Freeze-Drying | High porosities (ca. 98%) | Small-scale and time-consuming production | [37,54] |
| High interconnectivity of the porous network | High energy consumption | ||
| Channel-like pores and anisotropic structure | Use of cytotoxic organic solvents | ||
| Tunable pore size and structure | High sublimation time required | ||
| Capability of integrating bioactive molecules | Typical tissue shrinkage | ||
| Electrospinning | Wide range of polymers (synthetic and natural) | Limitations to produce 3D scaffolds | [122,123] |
| It produces continuous fiber on a micro-nano scale | Some of the solvents used can be cytotoxic | ||
| Control over fiber diameters and orientation | Poor control on pore size and shape | ||
| Versatile and well characterized technique | |||
| 3D Printing technologies | Advantages | Drawbacks | References |
| Stereolithography (SLA) | High resolution | Large number of monomers (resin) required | [124] |
| Excess liquid can be relatively easily removed | Low range of materials for photopolymerization | ||
| Uniformity in pores and interconnectivity | A post-polymerization stage is typically required | ||
| Fused Deposition Modeling (FDM) | High cost-effective processing | Limited to regular and simple porous structures | |
| It allows the use of multiple nozzles | Low utility with non-thermoplastic polymers | [125,126] | |
| Suitable for the design and manufacture of scaffolds | Little application with biodegradable polymers | ||
| It allows deposition at moderate temperature | |||
| Selective Laser Sintering (SLS) | Low operating cost | High operating temperatures are reached | [127,128] |
| Excellent control of the scaffold microstructure | Complex removal of excess material | ||
| Suitable with ceramics, metals and composites | A post-sinter stage required | ||
| Binder Jetting (BJ) 3D Printing | Relatively low operating cost | Small range of suitable polymers and binders | [129,130] |
| It allows complex morphologies with good precision | Complex removal of excess material | ||
| Suitable for incorporating cells into the scaffold | Contractions and deformations of scaffolds | ||
| Post-processing stage at high temperature |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-Based Scaffolds for Soft-Tissue Engineering. Polymers 2020, 12, 1566. https://doi.org/10.3390/polym12071566
Perez-Puyana V, Jiménez-Rosado M, Romero A, Guerrero A. Polymer-Based Scaffolds for Soft-Tissue Engineering. Polymers. 2020; 12(7):1566. https://doi.org/10.3390/polym12071566
Chicago/Turabian StylePerez-Puyana, Victor, Mercedes Jiménez-Rosado, Alberto Romero, and Antonio Guerrero. 2020. "Polymer-Based Scaffolds for Soft-Tissue Engineering" Polymers 12, no. 7: 1566. https://doi.org/10.3390/polym12071566