Adsorption Behavior of Polyelectrolyte onto Alumina and Application in Ciprofloxacin Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Studies
2.3. Analysis and Characterization
2.4. Modeling by General Isotherm Equation
2.5. Adsorption Kinetic
3. Results and Discussion
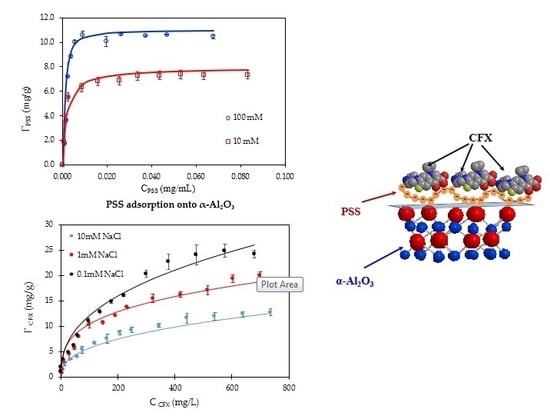
3.1. Adsorption of PSS onto α-Al2O3
3.2. Optimization of Effective Parameters for CFX-Removal
3.2.1. Effect of pH on CFX-Removal Using α-Al2O3 and PSS-Modified α-Al2O3 (PMA)
3.2.2. Effect of Ionic Strength on CFX-Removal Using α-Al2O3 and PMA
3.2.3. Effect of Contact Time on CFX-Removal Using PMA
3.2.4. Effect of Adsorption Dosage on CFX-Removal Using PMA
3.3. Adsorption Isotherms of CFX onto PMA
3.4. Adsorption Kinetic of CFX onto PMA
3.5. Regeneration Study
3.6. Application for CFX-Removal from Hospital Wastewater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pendhari, S.S.; Kant, T.; Desai, Y.M. Application of polymer composites in civil construction: A general review. Compos. Struct. 2008, 84, 114–124. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—a review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered composite based on halloysite and natural polymers: A carrier for the pH controlled release of drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef] [Green Version]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Biopolymer-Targeted Adsorption onto Halloysite Nanotubes in Aqueous Media. Langmuir ACS J. Surf. Colloids 2017, 33, 3317–3323. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Dao, T.H.; Truong, T.T.; Nguyen, T.M.T.; Pham, T.D. Adsorption characteristic of ciprofloxacin antibiotic onto synthesized alpha alumina nanoparticles with surface modification by polyanion. J. Mol. Liq. 2020, 309, 113150. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Walsh, T.R.; Efthimiou, J.; Dréno, B. Systematic review of antibiotic resistance in acne: An increasing topical and oral threat. Lancet Infect. Dis. 2016, 16, e23–e33. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Modarai, M.; Naylor, N.R.; Boyd, S.E.; Atun, R.; Barlow, J.; Holmes, A.H.; Johnson, A.; Robotham, J.V. Quantifying drivers of antibiotic resistance in humans: A systematic review. Lancet Infect. Dis. 2018, 18, e368–e378. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Song, W.; Lin, H.; Wang, W.; Du, L.; Xing, W. Antibiotics and antibiotic resistance genes in global lakes: A review and meta-analysis. Environ. Int. 2018, 116, 60–73. [Google Scholar] [CrossRef]
- Yahya, M.S.; Oturan, N.; El Kacemi, K.; El Karbane, M.; Aravindakumar, C.; Oturan, M.A. Oxidative degradation study on antimicrobial agent ciprofloxacin by electro-Fenton process: Kinetics and oxidation products. Chemosphere 2014, 117, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Trovo, A.G.; Nogueira, R.F.P.; Agüera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the antibiotic amoxicillin by photo-Fenton process–chemical and toxicological assessment. Water Res. 2011, 45, 1394–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvin, S.; Boyle, F.; Hickey, P.; Vellinga, A.; Morris, D.; Cormican, M. Enumeration and characterization of antimicrobial-resistant Escherichia coli bacteria in effluent from municipal, hospital, and secondary treatment facility sources. Appl. Environ. Microbiol. 2010, 76, 4772–4779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautitz, I.R.; Nogueira, R.F.P. Degradation of tetracycline by photo-Fenton process—Solar irradiation and matrix effects. J. Photochem. Photobiol. A Chem. 2007, 187, 33–39. [Google Scholar] [CrossRef]
- Elmolla, E.; Chaudhuri, M. Optimization of Fenton process for treatment of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution. J. Hazard. Mater. 2009, 170, 666–672. [Google Scholar] [CrossRef]
- Dao, T.H.; Tran, T.T.; Nguyen, V.R.; Pham, T.N.M.; Vu, C.M.; Pham, T.D. Removal of antibiotic from aqueous solution using synthesized TiO 2 nanoparticles: Characteristics and mechanisms. Environ. Earth Sci. 2018, 77, 359. [Google Scholar] [CrossRef]
- Chu, T.P.M.; Nguyen, N.T.; Vu, T.L.; Dao, T.H.; Dinh, L.C.; Nguyen, H.L.; Hoang, T.H.; Le, T.S.; Pham, T.D. Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal. Materials 2019, 12, 450. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.D.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Pham, T.D.; Do, T.U.; Pham, T.T.; Nguyen, T.A.H.; Nguyen, T.K.T.; Vu, N.D.; Le, T.S.; Vu, C.M.; Kobayashi, M. Adsorption of poly (styrenesulfonate) onto different-sized alumina particles: Characteristics and mechanisms. Colloid Polym. Sci. 2019, 297, 13–22. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Qiao, G.-L.; Zhao, F.; Wang, Y. Thermodynamic and kinetic parameters of ciprofloxacin adsorption onto modified coal fly ash from aqueous solution. J. Mol. Liq. 2011, 163, 53–56. [Google Scholar] [CrossRef]
- Li, M.-F.; Liu, Y.-G.; Liu, S.-B.; Zeng, G.-M.; Hu, X.-J.; Tan, X.-F.; Jiang, L.-H.; Liu, N.; Wen, J.; Liu, X.-H. Performance of magnetic graphene oxide/diethylenetriaminepentaacetic acid nanocomposite for the tetracycline and ciprofloxacin adsorption in single and binary systems. J. Colloid Interface Sci. 2018, 521, 150–159. [Google Scholar] [CrossRef]
- Zhu, B.-Y.; Gu, T. Surfactant adsorption at solid-liquid interfaces. Adv. Colloid Interface Sci. 1991, 37, 1–32. [Google Scholar] [CrossRef]
- Pham, T.D.; Pham, T.T.; Phan, M.N.; Ngo, T.M.V.; Dang, V.D.; Vu, C.M. Adsorption characteristics of anionic surfactant onto laterite soil with differently charged surfaces and application for cationic dye removal. J. Mol. Liq. 2020, 301, 112456. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption of anionic surfactant sodium dodecyl sulfate onto alpha alumina with small surface area. Colloid Polym. Sci. 2015, 293, 217–227. [Google Scholar] [CrossRef]
- Pham, T.D.; Vu, T.N.; Nguyen, H.L.; Le, P.H.P.; Hoang, T.S. Adsorptive Removal of Antibiotic Ciprofloxacin from Aqueous Solution Using Protein-Modified Nanosilica. Polymers 2020, 12, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption of Polyanion onto Large Alpha Alumina Beads with Variably Charged Surface. Adv. Phys. Chem. 2014, 2014, 9. [Google Scholar] [CrossRef]
- Mészáros, R.; Thompson, L.; Bos, M.; de Groot, P. Adsorption and Electrokinetic Properties of Polyethylenimine on Silica Surfaces. Langmuir 2002, 18, 6164–6169. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Zembala, M.; Warszyński, P.; Jachimska, B. Characterization of Polyelectrolyte Multilayers by the Streaming Potential Method. Langmuir 2004, 20, 10517–10525. [Google Scholar] [CrossRef]
- Guzmán, E.; Cavallo, J.A.; Chuliá-Jordán, R.; Gómez, C.; Strumia, M.C.; Ortega, F.; Rubio, R.G. pH-Induced Changes in the Fabrication of Multilayers of Poly(acrylic acid) and Chitosan: Fabrication, Properties, and Tests as a Drug Storage and Delivery System. Langmuir 2011, 27, 6836–6845. [Google Scholar] [CrossRef]
- Guzman, E.; Ritacco, H.; Rubio, J.E.F.; Rubio, R.G.; Ortega, F. Salt-induced changes in the growth of polyelectrolyte layers of poly(diallyl-dimethylammonium chloride) and poly(4-styrene sulfonate of sodium). Soft Matter 2009, 5, 2130–2142. [Google Scholar] [CrossRef]
- Hoffmann, I.; Oppel, C.; Gernert, U.; Barreleiro, P.; von Rybinski, W.; Gradzielski, M. Adsorption Isotherms of Cellulose-Based Polymers onto Cotton Fibers Determined by Means of a Direct Method of Fluorescence Spectroscopy. Langmuir 2012, 28, 7695–7703. [Google Scholar] [CrossRef] [PubMed]
- Moujahid, E.M.; Inacio, J.; Besse, J.-P.; Leroux, F. Adsorption of styrene sulfonate vs. polystyrene sulfonate on layered double hydroxides. Microporous Mesoporous Mater. 2003, 57, 37–46. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Çetinkaya, A. Electrokinetic and rheological properties of kaolinite in poly (diallyldimethylammonium chloride), poly (sodium 4-styrene sulfonate) and poly (vinyl alcohol) solutions. Colloids Surf. A Physicochem. Eng. Asp. 2012, 394, 23–32. [Google Scholar] [CrossRef]
- Genç, N.; Dogan, E.C. Adsorption kinetics of the antibiotic ciprofloxacin on bentonite, activated carbon, zeolite, and pumice. Desalin. Water Treat. 2015, 53, 785–793. [Google Scholar] [CrossRef]
- El-Shafey, E.-S.I.; Al-Lawati, H.; Al-Sumri, A.S. Ciprofloxacin adsorption from aqueous solution onto chemically prepared carbon from date palm leaflets. J. Environ. Sci. 2012, 24, 1579–1586. [Google Scholar] [CrossRef]
- Aloulou, F.; Boufi, S.; Beneventi, D. Adsorption of organic compounds onto polyelectrolyte immobilized-surfactant aggregates on cellulosic fibers. J. Colloid Interface Sci. 2004, 280, 350–358. [Google Scholar] [CrossRef]
- Pham, T.; Bui, T.; Nguyen, V.; Bui, T.; Tran, T.; Phan, Q.; Hoang, T. Adsorption of polyelectrolyte onto nanosilica synthesized from rice husk: Characteristics, mechanisms, and application for antibiotic removal. Polymers 2018, 10, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.D.; Nguyen, H.H.; Nguyen, N.V.; Vu, T.T.; Pham, T.N.M.; Doan, T.H.Y.; Nguyen, M.H.; Ngo, T.M.V. Adsorptive removal of copper by using surfactant modified laterite soil. J. Chem. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]










| C NaCl (mM) | k1,PSS (g/mg) | k2,PSS (g/mg)n−1 | nPSS | |
|---|---|---|---|---|
| 100 | 11.0 | 900 | 1000 | 1.9 |
| 10 | 8.0 | 1000 | 150 | 1.9 |
| C NaCl (mM) | k1,CFX (104 g/mg) | k2,CFX (g/mg)n−1 | nCFX | |
|---|---|---|---|---|
| 0.1 | 28.02 | 22 | 1260 | 1.4 |
| 1 | 19.92 | 9 | 1249 | 1.4 |
| 10 | 13.02 | 2 | 1250 | 1.4 |
| Ci (mg/L) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| K1,k (1/min) | qe (mg/g) | R2 | K2,k (g/mg.min) | qe (mg/g) | R2 | |
| 10 | 0.146 | 1.835 | 0.9863 | 0.225 | 1.904 | 0.9990 |
| 50 | 0.131 | 4.879 | 0.9984 | 0.106 | 4.930 | 0.9991 |
| 250 | 0.124 | 13.865 | 0.9686 | 0.014 | 14.992 | 0.9969 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dao, T.H.; Nguyen, N.T.; Nguyen, M.N.; Ngo, C.L.; Luong, N.H.; Le, D.B.; Pham, T.D. Adsorption Behavior of Polyelectrolyte onto Alumina and Application in Ciprofloxacin Removal. Polymers 2020, 12, 1554. https://doi.org/10.3390/polym12071554
Dao TH, Nguyen NT, Nguyen MN, Ngo CL, Luong NH, Le DB, Pham TD. Adsorption Behavior of Polyelectrolyte onto Alumina and Application in Ciprofloxacin Removal. Polymers. 2020; 12(7):1554. https://doi.org/10.3390/polym12071554
Chicago/Turabian StyleDao, Thi Huong, Ngoc Trung Nguyen, Minh Ngoc Nguyen, Cao Long Ngo, Nhu Hai Luong, Duy Binh Le, and Tien Duc Pham. 2020. "Adsorption Behavior of Polyelectrolyte onto Alumina and Application in Ciprofloxacin Removal" Polymers 12, no. 7: 1554. https://doi.org/10.3390/polym12071554