Light Processable Starch Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
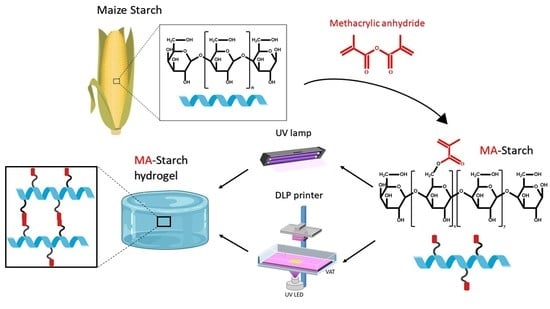
2.2. Synthesis of Methacrylated Starch
2.3. Photocuring of Methacrylated Starch
2.4. 3D-Printing of Methacrylated Starch
2.5. Nuclear Magnetic Resonance (NMR)
2.6. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.7. Photorheology and Rheology
2.8. Compression Test
2.9. Swelling Behavior
2.10. Gel Content
2.11. Differential Scanning Calorimetry (DSC)
2.12. Cell Viability
3. Results and Discussion
3.1. Confirmation of Starch Methacrylation
3.2. Photo-Rheology of the Photo-Crosslinking Reaction for Forming Starch Hydrogels
3.3. Characterization of the Photo-Crosslinked Starch Hydrogels
3.4. Cell Viability of the Photo-Crosslinked Starch Hydrogels
3.5. The 3D Printability of the Methacrylated Starch Hydrogels by DLP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Dai, T.; Xu, P.; Wu, P.; Zou, Y.; Yang, P.; Hu, J.; Li, Y.; Cheng, Y. Skin Pigmentation-Inspired Polydopamine Sunscreens. Adv. Funct. Mater. 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Hu, J.; Hu, J.; Zhang, S.; Yang, Z.; Li, Y.; Cheng, Y. Smart Hydrogels with Antibacterial Properties Built from All Natural Building Blocks. Chem. Mater. 2019, 31, 7678–7685. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Piluso, S.; Labet, M.; Zhou, C.; Seo, J.W.; Thielemans, W.; Patterson, J. Engineered Three-Dimensional Microenvironments with Starch Nanocrystals as Cell-Instructive Materials. Biomacromolecules 2019, 20, 3819–3830. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Danjo, T.; Odelius, K.; Hakkarainen, M.; Iwata, T.; Albertsson, A.C. Recyclable Fully Biobased Chitosan Adsorbents Spray-Dried in One Pot to Microscopic Size and Enhanced Adsorption Capacity. Biomacromolecules 2019, 20, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Morits, M.; Jonkergouw, C.; Ora, A.; Valle-Delgado, J.J.; Farooq, M.; Ajdary, R.; Huan, S.; Linder, M.; Rojas, O.; et al. Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel. Biomacromolecules 2020, 21, 1875–1885. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.; Salamon, A.; Peters, K.; Graulus, G.J.; Martins, J.C.; Frankel, D.; Kersemans, K.; De Vos, F.; Van Vlierberghe, S.; Dubruel, P. Gelatin- and starch-based hydrogels. Part A: Hydrogel development, characterization and coating. Carbohydr. Polym. 2016, 152, 129–139. [Google Scholar] [CrossRef]
- Szepes, A.; Makai, Z.; Blümer, C.; Mäder, K.; Kása, P.; Szabó-Révész, P. Characterization and drug delivery behaviour of starch-based hydrogels prepared via isostatic ultrahigh pressure. Carbohydr. Polym. 2008, 72, 571–578. [Google Scholar] [CrossRef]
- Kulicke, W.-M.; Aggour, Y.A.; Nottelmann, H.; Elsabee, M.Z. Swelling and Rheological Studies of Some Starch Hydrogels. Starch Stärke 1989, 41, 140–146. [Google Scholar] [CrossRef]
- Song, D.; Breedveld, V.; Deng, Y. Rheological study of self-crosslinking and co-crosslinking of ammonium zirconium carbonate and starch in aqueous solutions. J. Appl. Polym. Sci. 2011, 122, 1019–1029. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-curing: Technology and applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Messori, M. UV-cured functional coatings. RSC Smart Mater. 2015, 2015, 121–133. [Google Scholar]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New horizons in cationic photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Hakkarainen, M.; Grützmacher, H.; Chiappone, A.; Sangermano, M. Photocrosslinked Chitosan Hydrogels Reinforced with Chitosan-Derived Nano-Graphene Oxide. Macromol. Chem. Phys. 2019, 220, 1–6. [Google Scholar] [CrossRef]
- Tonda-turo, C.; Carmagnola, I.; Chiappone, A.; Feng, Z. Bioprinting Photocurable chitosan as bioink for cellularized therapies towards personalized scaffold architecture. Bioprinting 2020, 18, e00082. [Google Scholar] [CrossRef]
- Han, T.L.; Kumar, R.N.; Rozman, H.D.; Noor, M.A.M. GMA grafted sago starch as a reactive component in ultra violet radiation curable coatings. Carbohydr. Polym. 2003, 54, 509–516. [Google Scholar] [CrossRef]
- Athawale, V.D.; Lele, V. Graft copolymerization onto starch. II. Grafting of acrylic acid and preparation of it’s hydrogels. Carbohydr. Polym. 1998, 35, 21–27. [Google Scholar] [CrossRef]
- Lee, J.S.; Kumar, R.N.; Rozman, H.D.; Azemi, B.M.N. Pasting, swelling and solubility properties of UV initiated starch-graft-poly(AA). Food Chem. 2005, 91, 203–211. [Google Scholar] [CrossRef]
- Saboktakin, M.R.; Maharramov, A.; Ramazanov, M.A. pH-sensitive starch hydrogels via free radical graft copolymerization, synthesis and properties. Carbohydr. Polym. 2009, 77, 634–638. [Google Scholar] [CrossRef]
- Nabais, T.; Brouillet, F.; Kyriacos, S.; Mroueh, M.; da Amores Silva, P.; Bataille, B.; Chebli, C.; Cartilier, L. High-amylose carboxymethyl starch matrices for oral sustained drug-release: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2007, 65, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Lee, S.J. Characteristics of crosslinked potato starch and starch-filled linear low-density polyethylene films. Carbohydr. Polym. 2002, 50, 331–337. [Google Scholar] [CrossRef]
- Khan, M.A.; Bhattacharia, S.K.; Kader, M.A.; Bahari, K. Preparation and characterization of ultra violet (UV) radiation cured bio-degradable films of sago starch/PVA blend. Carbohydr. Polym. 2006, 63, 500–506. [Google Scholar] [CrossRef]
- Khan, M.A.; Rahman, M.A.; Khan, R.A.; Rahman, N.; Islam, J.M.M.; Alam, R.; Mondal, M.I.H. Preparation and characterization of the mechanical properties of the photocured chitosan/starch blend film. Polym. Plast. Technol. Eng. 2010, 49, 748–756. [Google Scholar] [CrossRef]
- Delville, J.; Joly, C.; Dole, P.; Bliard, C. Solid state photocrosslinked starch based films: A new family of homogeneous modified starches. Carbohydr. Polym. 2002, 49, 71–81. [Google Scholar] [CrossRef]
- Zain, A.H.M.; Wahab, M.K.A.; Ismail, H. Solid-state photo-cross-linking of cassava starch: Improvement properties of thermoplastic starch. Polym. Bull. 2018, 75, 3341–3356. [Google Scholar] [CrossRef]
- Reis, A.V.; Guilherme, M.R.; Moia, T.A.; Mattoso, L.H.C.; Muniz, E.C.; Tambourgi, E.B. Synthesis and characterization of a starch-modified hydrogel as potential carrier for drug delivery system. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2567–2574. [Google Scholar] [CrossRef]
- Hedin, J.; Östlund, Å.; Nydén, M. UV induced cross-linking of starch modified with glycidyl methacrylate. Carbohydr. Polym. 2010, 79, 606–613. [Google Scholar] [CrossRef]
- Li, J.M.; Zhang, L.M. Characteristics of novel starch-based hydrogels prepared by UV photopolymerization of acryloylated starch and a zwitterionic monomer. Starch Staerke 2007, 59, 418–422. [Google Scholar] [CrossRef]
- Harling, S.; Schwoerer, A.; Scheibe, K.; Daniels, R.; Menzel, H. A new hydrogel drug delivery system based on Hydroxyethylstarch derivatives. J. Microencapsul. 2010, 27, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Wöhl-Bruhn, S.; Bertz, A.; Harling, S.; Menzel, H.; Bunjes, H. Hydroxyethyl starch-based polymers for the controlled release of biomacromolecules from hydrogel microspheres. Eur. J. Pharm. Biopharm. 2012, 81, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhove, I.; Salamon, A.; Adam, S.; Dubruel, P.; Van Vlierberghe, S.; Peters, K. Gelatin- and starch-based hydrogels. Part B: In vitro mesenchymal stem cell behavior on the hydrogels. Carbohydr. Polym. 2017, 161, 295–305. [Google Scholar] [CrossRef]
- Arno, M.C.; Dove, A.P.; Weems, A.C.; Pe, M.M. 3D Printing for the Clinic: Examining Contemporary Polymeric Biomaterials and Their Clinical Utility. Biomacromolecules 2020, 21, 1037–1059. [Google Scholar] [CrossRef]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, 1902026. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.T.; Narupai, B.; Tsui, J.H.; Millik, S.C.; Shafranek, R.T.; Kim, D.H.; Nelson, A. Additive Manufacturing of Bovine Serum Albumin-Based Hydrogels and Bioplastics. Biomacromolecules 2020, 21, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Le Fer, G.; Dean, D.; Becker, M.L. 3D Printing of Poly(propylene fumarate) Oligomers: Evaluation of Resin Viscosity, Printing Characteristics and Mechanical Properties. Biomacromolecules 2019, 20, 1699–1708. [Google Scholar] [CrossRef]
- Lawal, O.S.; Storz, J.; Storz, H.; Lohmann, D.; Lechner, D.; Kulicke, W.M. Hydrogels based on carboxymethyl cassava starch cross-linked with di- or polyfunctional carboxylic acids: Synthesis, water absorbent behavior and rheological characterizations. Eur. Polym. J. 2009, 45, 3399–3408. [Google Scholar] [CrossRef]
- Murthy, P.S.K.; Mohan, Y.M.; Sreeramulu, J.; Raju, K.M. Semi-IPNs of starch and poly(acrylamide-co-sodium methacrylate): Preparation, swelling and diffusion characteristics evaluation. React. Funct. Polym. 2006, 66, 1482–1493. [Google Scholar] [CrossRef]
- Dragan, E.S.; Apopei, D.F. Synthesis and swelling behavior of pH-sensitive semi-interpenetrating polymer network composite hydrogels based on native and modified potatoes starch as potential sorbent for cationic dyes. Chem. Eng. J. 2011, 178, 252–263. [Google Scholar] [CrossRef]
- Wu, D.; Samanta, A.; Srivastava, R.K.; Hakkarainen, M. Starch-Derived Nanographene Oxide Paves the Way for Electrospinnable and Bioactive Starch Scaffolds for Bone Tissue Engineering. Biomacromolecules 2017, 18, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hakkarainen, M. A closed-loop process from microwave-assisted hydrothermal degradation of starch to utilization of the obtained degradation products as starch plasticizers. ACS Sustain. Chem. Eng. 2014, 2, 2172–2181. [Google Scholar] [CrossRef]
- Bayramoglu, G. Methacrylated chitosan based UV curable support for enzyme immobilization. Mater. Res. 2017, 20, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Hajirahimkhan, S.; Ragogna, P.J.; Xu, C. (Charles) Methacrylation of kraft lignin for UV-curable coatings: Process optimization using response surface methodology. Biomass Bioenergy 2019, 120, 332–338. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Yang, P.; Långvik, O.; Wang, X.; Zhang, Y.; Cheng, F.; Österberg, M.; Willför, S.; Xu, C. Surface Engineered Biomimetic Inks Based on UV Cross-Linkable Wood Biopolymers for 3D Printing. ACS Appl. Mater. Interfaces 2019, 11, 12389–12400. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef]
- Han, F.; Zhu, C.; Guo, Q.; Yang, H.; Li, B. Cellular modulation by the elasticity of biomaterials. J. Mater. Chem. B 2016, 4, 9–26. [Google Scholar] [CrossRef]
- Seidel, C.; Kulicke, W.M.; Heß, C.; Hartmann, B.; Lechner, M.D.; Lazik, W. Synthesis and characterization of cross-linked carboxymethyl potato starch ether gels. Starch Staerke 2004, 56, 157–166. [Google Scholar] [CrossRef]
- Seidel, C.; Kulicke, W.M.; Heß, C.; Hartmann, B.; Lechner, M.D.; Lazik, W. Influence of the cross-linking agent on the gel structure of starch derivatives. Starch Staerke 2001, 53, 305–310. [Google Scholar] [CrossRef]
- Kowalski, G.; Ptaszek, P. The effect of swelling time on rheological properties of hydrogels, consisting of high -amylose carboxymethyl corn starch and acrylic polymers. Starch Staerke 2016, 68, 381–388. [Google Scholar] [CrossRef]
- Raja, C.; Pratap, K.; Saikat, S. Natural Starch in and Food Industry: Perception and Overview. Curr. Drug Discov. Technol. 2019, 16, 355–367. [Google Scholar] [CrossRef]
- Wang, J.; Chiappone, A.; Roppolo, I.; Shao, F.; Fantino, E.; Lorusso, M.; Rentsch, D.; Dietliker, K.; Pirri, C.F.; Grützmacher, H. All-in-One Cellulose Nanocrystals for 3D Printing of Nanocomposite Hydrogels. Angew. Chem. 2018, 130, 2377–2380. [Google Scholar] [CrossRef]
- Wu, D.; Bäckström, E.; Hakkarainen, M. Starch Derived Nanosized Graphene Oxide Functionalized Bioactive Porous Starch Scaffolds. Macromol. Biosci. 2017, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Frascella, F.; González, G.; Bosch, P.; Angelini, A.; Chiappone, A.; Sangermano, M.; Pirri, C.F.; Roppolo, I. Three-Dimensional Printed Photoluminescent Polymeric Waveguides. ACS Appl. Mater. Interfaces 2018, 10, 39319–39326. [Google Scholar] [CrossRef]








| Hydrogel | Starch | MA-Starch | MA-Starch5 | MA-Starch10 | MA-Starch15 |
|---|---|---|---|---|---|
| Ec [kPa] | - | - | - | 12.7 ± 0.4 | 20.3 ± 1.7 |
| G’P [Pa] | - | - | 105 | 159 | 178 |
| νe [m−3] | - | - | 2.55 × 1022 | 3.86 × 1022 | 4.32 × 1022 |
| ξ [m] | - | - | 3.40 × 10−8 | 2.96 × 10−8 | 2.85 × 10−8 |
| Seq | - | - | 3.6 * | 2.7 | 3.6 |
| EWC% | - | - | 78 * | 78 | 73 |
| G% | - | - | 100% | 100% | 100% |
| Tg (°C) | 105 ± 2 | 115 ± 1 | 106 ± 2 | 106 ± 9 | 101 ± 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noè, C.; Tonda-Turo, C.; Chiappone, A.; Sangermano, M.; Hakkarainen, M. Light Processable Starch Hydrogels. Polymers 2020, 12, 1359. https://doi.org/10.3390/polym12061359
Noè C, Tonda-Turo C, Chiappone A, Sangermano M, Hakkarainen M. Light Processable Starch Hydrogels. Polymers. 2020; 12(6):1359. https://doi.org/10.3390/polym12061359
Chicago/Turabian StyleNoè, Camilla, Chiara Tonda-Turo, Annalisa Chiappone, Marco Sangermano, and Minna Hakkarainen. 2020. "Light Processable Starch Hydrogels" Polymers 12, no. 6: 1359. https://doi.org/10.3390/polym12061359