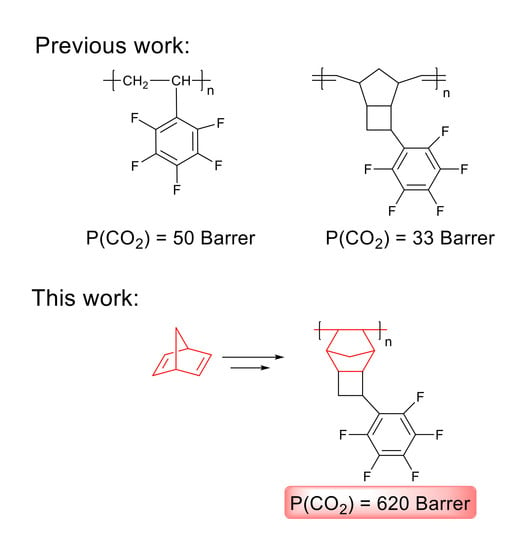
Synthesis and Gas Transport Properties of Addition Polynorbornene with Perfluorophenyl Side Groups
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods of Polymer Characterization
2.3. Film Preparation
2.4. Measurements of Gas Transport Properties
2.5. Addition Polymerization of 3-Perfluorophenyltricyclononene-7
3. Results and Discussion
3.1. Synthesis of the Polymer
3.2. Physico-Chemical Properties
3.3. Gas Transport Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Starannikova, L.; Belov, N.; Bermeshev, M.; Gringolts, M.; Finkelshtein, E. Solubility controlled permeation of hydrocarbons: New membrane materials and results. J. Membr. Sci. 2014, 453, 532–545. [Google Scholar] [CrossRef]
- Nagai, K.; Masuda, T.; Nakagawa, T.; Freeman, B.D.; Pinnau, I. Poly[1-(trimethylsilyl)-1-propyne] and related polymers: Synthesis, properties and functions. Prog. Polym. Sci. 2001, 26, 721–798. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.M. Rigid and microporous polymers for gas separation membranes. Prog. Polym. Sci. 2015, 43, 1–32. [Google Scholar] [CrossRef]
- Jue, M.L.; Lively, R.P. Targeted gas separations through polymer membrane functionalization. React. Funct. Polym. 2015, 86, 88–110. [Google Scholar] [CrossRef]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas Permeation Properties, Physical Aging, and Its Mitigation in High Free Volume Glassy Polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef]
- Finkelshtein, E.; Gringolts, M.; Bermeshev, M.; Chapala, P.; Rogan, Y. Polynorbornenes. In Membrane Materials for Gas and Vapor Separation; Yampolskii, Y., Finkelshtein, E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 143–221. [Google Scholar]
- Yevlampieva, N.P.; Bermeshev, M.V.; Komolkin, A.V.; Vezo, O.S.; Chapala, P.P.; Il’yasova, Y.V. The equilibrium and kinetic rigidity of additive poly(trimethylsilyltricyclononenes) with one and two Si(CH3)3 groups in monomer unit. Polym. Sci. Ser. A 2017, 59, 473–482. [Google Scholar] [CrossRef]
- Yevlampieva, N.P.; Bermeshev, M.V.; Vezo, O.S.; Bermesheva, E.V.; Voznyak, A.I.; Kim, R.O. Synthesis and Molecular Properties of Additive Poly(5-ethylidene-2-norbornene). Polym. Sci. Ser. A 2019, 61, 134–141. [Google Scholar] [CrossRef]
- Bermeshev, M.V.; Chapala, P.P. Addition polymerization of functionalized norbornenes as a powerful tool for assembling molecular moieties of new polymers with versatile properties. Prog. Polym. Sci. 2018, 84, 1–46. [Google Scholar] [CrossRef]
- Bermesheva, E.V.; Alentiev, D.A.; Moskalets, A.P.; Bermeshev, M.V. New Adhesive Materials Based on Silicon-Substituted Polynorbornenes. Polym. Sci. Ser. B 2019, 61, 314–322. [Google Scholar] [CrossRef]
- Kim, T.; Lim, S.; Park, S.-R.; Han, C.J.; Lee, M.H. Polynorbornene copolymer with side-chain triarylborane and iridium(III) groups: An emissive layer material with electron transporting properties for PhOLEDs. Polymer 2015, 66, 67–75. [Google Scholar] [CrossRef]
- Park, J.H.; Yun, C.; Koh, T.-W.; Do, Y.; Yoo, S.; Lee, M.H. Vinyl-type polynorbornene with 9,9[prime or minute]-(1,1[prime or minute]-biphenyl)-4,4[prime or minute]-diylbis-9H-carbazole side groups as a host material for highly efficient green phosphorescent organic light-emitting diodes. J. Mater. Chem. 2011, 21, 5422–5429. [Google Scholar] [CrossRef]
- Park, J.H.; Yun, C.; Park, M.H.; Do, Y.; Yoo, S.; Lee, M.H. Vinyl-Type Polynorbornenes with Triarylamine Side Groups: A New Class of Soluble Hole-Transporting Materials for OLEDs. Macromolecules 2009, 42, 6840–6843. [Google Scholar] [CrossRef]
- García-Loma, R.; Albéniz, A.C. Vinylic Addition Polynorbornene in Catalysis. Asian J. Org. Chem. 2019, 8, 304–315. [Google Scholar] [CrossRef]
- Molina de la Torre, J.A.; Albéniz, A.C. α-Diimine–Palladium Complexes Incorporated in Vinylic-Addition Polynorbornenes: Synthesis and Catalytic Activity. Eur. J. Inorg. Chem. 2017, 2017, 2911–2919. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.-G.; Kim, D.-G.; Register, R.A. Vinyl Addition Copolymers of Norbornylnorbornene and Hydroxyhexafluoroisopropylnorbornene for Efficient Recovery of n-Butanol from Dilute Aqueous Solution via Pervaporation. Macromolecules 2018, 51, 3702–3710. [Google Scholar] [CrossRef]
- He, X.; Jiang, X.; Wang, Z.; Deng, Y.; Han, Z.; Yang, Y.; Chen, D. Cross-linked hydroxyl-conductive copolymer/silica composite membranes based on addition-type polynorbornene for alkaline anion exchange membrane fuel cell applications. Polym. Eng. Sci. 2017, 58, 13–21. [Google Scholar] [CrossRef]
- Alentiev, D.A.; Dzhaparidze, D.M.; Gavrilova, N.N.; Shantarovich, V.P.; Kiseleva, E.V.; Topchiy, M.A.; Asachenko, A.F.; Gribanov, P.S.; Nechaev, M.S.; Legkov, S.A.; et al. Microporous Materials Based on Norbornadiene-Based Cross-Linked Polymers. Polymers 2018, 10, 1382. [Google Scholar] [CrossRef] [Green Version]
- Shamiryan, D.; Abell, T.; Iacopi, F.; Maex, K. Low-k dielectric materials. Mater. Today 2004, 7, 34–39. [Google Scholar] [CrossRef]
- Grove, N.R.; Kohl, P.A.; Bidstrup-Allen, S.A.; Shick, R.A.; Goodall, B.L.; Jayaraman, S. Polynorbornene for Low K Interconnection. MRS Proc. 1997, 476, 3–8. [Google Scholar] [CrossRef]
- You, Z.; Song, W.; Zhang, S.; Jin, O.; Xie, M. Polymeric microstructures and dielectric properties of polynorbornenes with 3,5-bis(trifluoromethyl)biphenyl side groups by ring-opening metathesis polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4786–4798. [Google Scholar] [CrossRef]
- Alentiev, D.A.; Egorova, E.S.; Bermeshev, M.V.; Starannikova, L.E.; Topchiy, M.A.; Asachenko, A.F.; Gribanov, P.S.; Nechaev, M.S.; Yampolskii, Y.P.; Finkelshtein, E.S. Janus tricyclononene polymers bearing tri(n-alkoxy)silyl side groups for membrane gas separation. J. Mater. Chem. A 2018, 6, 19393–19408. [Google Scholar] [CrossRef]
- Chapala, P.P.; Bermeshev, M.V.; Starannikova, L.E.; Belov, N.A.; Ryzhikh, V.E.; Shantarovich, V.P.; Lakhtin, V.G.; Gavrilova, N.N.; Yampolskii, Y.P.; Finkelshtein, E.S. A Novel, Highly Gas-Permeable Polymer Representing a New Class of Silicon-Containing Polynorbornens As Efficient Membrane Materials. Macromolecules 2015, 48, 8055–8061. [Google Scholar] [CrossRef]
- Maroon, C.R.; Townsend, J.; Higgins, M.A.; Harrigan, D.J.; Sundell, B.J.; Lawrence, J.A.; O’Brien, J.T.; O’Neal, D.; Vogiatzis, K.D.; Long, B.K. Addition-type alkoxysilyl-substituted polynorbornenes for post-combustion carbon dioxide separations. J. Membr. Sci. 2020, 595, 117532. [Google Scholar] [CrossRef]
- Dujardin, W.; Van Goethem, C.; Steele, J.A.; Roeffaers, M.; Vankelecom, I.F.J.; Koeckelberghs, G. Polyvinylnorbornene Gas Separation Membranes. Polymers 2019, 11, 704. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, A.I.; Bermesheva, E.V.; Borisov, I.L.; Petukhov, D.I.; Bermeshev, M.V.; Volkov, A.V.; Finkelshtein, E.S. Addition Polyalkylnorbornenes: A Promising New Class of Si-Free Membrane Materials for Hydrocarbons Separation. Macromol. Rapid Commun. 2019, 40, 1900206. [Google Scholar] [CrossRef]
- Wozniak, A.I.; Bermesheva, E.V.; Andreyanov, F.A.; Borisov, I.L.; Zarezin, D.P.; Bakhtin, D.S.; Gavrilova, N.N.; Ilyasov, I.R.; Nechaev, M.S.; Asachenko, A.F.; et al. Modifications of addition poly(5-vinyl-2-norbornene) and gas-transport properties of the obtained polymers. React. Funct. Polym. 2020, 104513. [Google Scholar] [CrossRef]
- Cruz-Morales, J.A.; Vargas, J.; Santiago, A.A.; Vásquez-García, S.R.; Tlenkopatchev, M.A.; Lys, T.d.; López-González, M. Synthesis and gas transport properties of new polynorbornene dicarboximides bearing trifluoromethyl isomer moieties. High Perform. Polym. 2016, 28, 1246–1262. [Google Scholar] [CrossRef]
- Tanaka, K.; Osada, Y.; Kita, H.; Okamoto, K.-I. Gas permeability and permselectivity of polyimides with large aromatic rings. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1907–1915. [Google Scholar] [CrossRef]
- Belov, N.; Nizhegorodova, Y.; Zharov, A.; Konovalova, I.; Shantarovich, V.; Yampolskii, Y. A new polymer, poly(perfluoropropylvinyl ether) and its comparison with other perfluorinated membrane materials. J. Membr. Sci. 2015, 495, 431–438. [Google Scholar] [CrossRef]
- Karpov, G.O.; Bermeshev, M.V.; Borisov, I.L.; Sterlin, S.R.; Tyutyunov, A.A.; Yevlampieva, N.P.; Bulgakov, B.A.; Volkov, V.V.; Finkelshtein, E.S. Metathesis-type poly-exo-tricyclononenes with fluoroorganic side substituents: Synthesis and gas-transport properties. Polymer 2018, 153, 626–636. [Google Scholar] [CrossRef]
- Yampol’skii, Y.P.; Bespalova, N.B.; Finkel’shtein, E.S.; Bondar, V.I.; Popov, A.V. Synthesis, Gas Permeability, and Gas Sorption Properties of Fluorine-Containing Norbornene Polymers. Macromolecules 1994, 27, 2872–2878. [Google Scholar] [CrossRef]
- Vargas, J.; Martínez, A.; Santiago, A.A.; Tlenkopatchev, M.A.; Aguilar-Vega, M. Synthesis and gas permeability of new polynorbornene dicarboximide with fluorine pendant groups. Polymer 2007, 48, 6546–6553. [Google Scholar] [CrossRef]
- Vargas, J.; Santiago, A.A.; Cruz-Morales, J.A.; Tlenkopatchev, M.A.; De Lys, T.; Lõpez-González, M.; Riande, E. Gas transport properties of hydrogenated and fluorinated polynorbornene dicarboximides. Macromol. Chem. Phys. 2013, 214, 2607–2615. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Neumann, S.; Bolmer, S.; Khan, M.M.; Abetz, V. PEBAX® with PEG functionalized POSS as nanocomposite membranes for CO2 separation. J. Membr. Sci. 2013, 437, 286–297. [Google Scholar] [CrossRef] [Green Version]
- Shishatskii, A.M.; Yampol’skii, Y.P.; Peinemann, K.V. Effects of film thickness on density and gas permeation parameters of glassy polymers. J. Membr. Sci. 1996, 112, 275–285. [Google Scholar] [CrossRef]
- Finkelshtein, E.S.; Makovetskii, K.L.; Gringolts, M.L.; Rogan, Y.V.; Golenko, T.G.; Starannikova, L.E.; Yampolskii, Y.P.; Shantarovich, V.P.; Suzuki, T. Addition-Type Polynorbornenes with Si(CH3)3 Side Groups: Synthesis, Gas Permeability, and Free Volume. Macromolecules 2006, 39, 7022–7029. [Google Scholar] [CrossRef]
- Belov, N.A.; Nikiforov, R.Y.; Bermeshev, M.V.; Yampolskii, Y.P.; Finkelshtein, E.S. Synthesis and gas transport properties of polypentafluorostyrene. Pet. Chem. 2017, 57, 923–928. [Google Scholar] [CrossRef]
- Gringol’ts, M.L.; Bermeshev, M.V.; Syromolotov, A.V.; Starannikova, L.E.; Filatova, M.F.; Makovetskii, K.L.; Finkel’shtein, E.S. Highly permeable polymer materials based on silicon-substituted norbornenes. Pet. Chem. 2010, 50, 352–361. [Google Scholar] [CrossRef]
- Nagai, K.; Toy, L.G.; Freeman, B.D.; Teraguchi, M.; Masuda, T.; Pinnau, I. Gas permeability and hydrocarbon solubility of poly[1-phenyl-2-[p-(triisopropylsilyl)phenyl]acetylene]. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1474–1484. [Google Scholar] [CrossRef]
- Yevlampieva, N.; Bermeshev, M.; Vezo, O.; Chapala, P.; Il’yasova, Y. Metathesis and additive poly(tricyclononenes) with geminal trimethylsilyl side groups: Chain rigidity, molecular and thin film properties. J. Polym. Res. 2018, 25, 162. [Google Scholar] [CrossRef]
- Zhao, C.-T.; do Rosário Ribeiro, M.; de Pinho, M.N.; Subrahmanyam, V.S.; Gil, C.L.; de Lima, A.P. Structural characteristics and gas permeation properties of polynorbornenes with retained bicyclic structure. Polymer 2001, 42, 2455–2462. [Google Scholar] [CrossRef] [Green Version]









| Monomer/Pd Molar Ratio | Yield of the Polymer, % |
|---|---|
| 3000:1 | traces |
| 1500:1 | 11 |
| 1000:1 | 36 |
| 500:1 | 67 |
| Monomer/Pd Molar Ratio | Monomer Concentration, M | Mw × 10−5 | Mn × 10−5 | Mw/Mn |
|---|---|---|---|---|
| 1500:1 | 1.2 | 4.10 | 1.12 | 3.6 |
| 1000:1 | 0.7 | 2.93 | 0.86 | 3.4 |
| 0.8 | 3.63 | 0.95 | 3.8 | |
| 1.2 | 4.10 | 1.10 | 3.7 | |
| 1.4 | 4.35 | 1.09 | 4.0 |
| Solvent | Polymer | |
|---|---|---|
| APF5 | MPF5 | |
| Dimethyl sulfoxide | - | - |
| Toluene | + | + |
| Chloroform | + | + |
| Tetrahydrofuran | + | + |
| Dimethyl formamide | - | - |
| Hexafluorobenzene | + | - |
| Octafluorotoluene | - | - |
| Hexane | - | - |
| 1,2,4-Trichlorobenzene | + | + |
| Polymer | (2θ)1 | d1-spacing, Å | (2θ)2 | d2-spacing, Å |
|---|---|---|---|---|
| APNB a [40] | 10.0 | 8.8 | 18.5 | 4.7 |
| MPF5 [34] | 18.0 | 4.9 | - | - |
| APF5 | 16.1 | 5.5 | - | - |
| Polymer | Gas | Ref. | |||||
|---|---|---|---|---|---|---|---|
| He | H2 | N2 | O2 | CO2 | CH4 | ||
| Permeability (P), Barrer | |||||||
| PPFS | 88 | 65 | 5.9 | 14 | 50 | 5.1 | [41] |
| MPF5 | 44 | 41 | 2.3 | 8.2 | 33 | 3.5 | [34] |
| APF5 | 400 | 300 | 48 | 130 | 620 | 55 | This work |
| Diffusivity coefficients D × 107, cm2/s | |||||||
| MPF5 | 200 | 53 | 1.0 | 2.3 | 0.92 | 0.35 | [34] |
| APF5 | 95 | 29 | 2.7 | 6.0 | 2.8 | 0.84 | This work |
| Solubility coefficients S × 104, cm3 (STP)/(cm3 × cm Hg) | |||||||
| MPF5 | 2.2 | 7.7 | 23 | 36 | 360 | 100 | [34] |
| APF5 | 42 | 100 | 180 | 220 | 2200 | 650 | This work |
| Polymer | Pairs of Gases | |||||
|---|---|---|---|---|---|---|
| O2/N2 | CO2/N2 | CO2/CH4 | H2/N2 | H2/CH4 | He/CH4 | |
| Permeability Selectivity | ||||||
| PPFS [41] | 2.4 | 8.5 | 10.0 | 11.0 | 12.7 | 17 |
| MPF5 [34] | 3.6 | 14.3 | 9.4 | 17.8 | 11.7 | 12.6 |
| APF5 | 2.7 | 12.9 | 11.7 | 6.3 | 5.5 | 7.3 |
| Diffusivity Selectivity | ||||||
| MPF5 [34] | 2.3 | 0.9 | 2.6 | 53 | 151 | 571 |
| APF5 | 2.2 | 1.0 | 3.3 | 10.7 | 34.5 | 113.1 |
| Solubility Selectivity | ||||||
| MPF5 [34] | 1.6 | 15.7 | 3.6 | 0.33 | 0.08 | 0.02 |
| APF5 | 1.2 | 12.2 | 3.4 | 0.56 | 0.15 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpov, G.O.; Borisov, I.L.; Volkov, A.V.; Finkelshtein, E.S.; Bermeshev, M.V. Synthesis and Gas Transport Properties of Addition Polynorbornene with Perfluorophenyl Side Groups. Polymers 2020, 12, 1282. https://doi.org/10.3390/polym12061282
Karpov GO, Borisov IL, Volkov AV, Finkelshtein ES, Bermeshev MV. Synthesis and Gas Transport Properties of Addition Polynorbornene with Perfluorophenyl Side Groups. Polymers. 2020; 12(6):1282. https://doi.org/10.3390/polym12061282
Chicago/Turabian StyleKarpov, Gleb O., Ilya L. Borisov, Alexey V. Volkov, Eugene Sh. Finkelshtein, and Maxim V. Bermeshev. 2020. "Synthesis and Gas Transport Properties of Addition Polynorbornene with Perfluorophenyl Side Groups" Polymers 12, no. 6: 1282. https://doi.org/10.3390/polym12061282