High-Molecular-Weight PLA-b-PEO-b-PLA Triblock Copolymer Templated Large Mesoporous Carbons for Supercapacitors and CO2 Capture
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
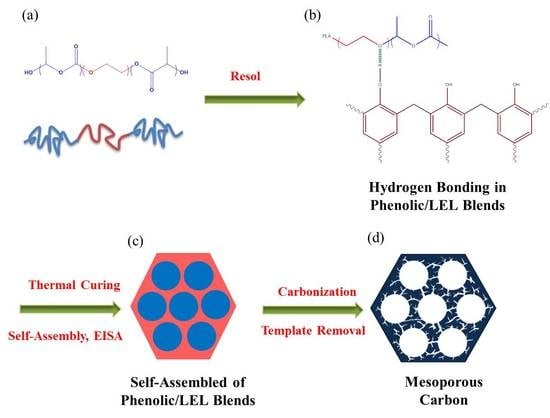
2.2. The Preparation of Mesoporous Carbon
3. Results and Discussion
3.1. Characterizations of Phenolic/PLA-b-PEO-b-PLA Blend
3.2. Analyses of Mesoporous Carbons from Phenolic/LEL Blends
3.3. Raman Spectra, CO2 Capture Ability, and Electrochemical Analyses of Mesoporous Carbons
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Xie, L.; Deng, J.; Gong, Y.; Tang, G.; Bai, H.; Wang, Y. Annular Mesoporous Carbonaceous Nanospheres from Biomass-Derived Building Units with Enhanced Biological Interactions. Chem. Mater. 2019, 31, 7186–7191. [Google Scholar] [CrossRef]
- Chu, W.C.; Kim, J.; Kim, M.; Alshehri, A.A.; Alghamidi, Y.G.; Alzahrani, K.A.; Yamauchi, Y.; Malgras, V.; Kuo, S.W. Photodegradation Activity of Poly (ethylene oxide-b-ε-caprolactone)-Templated Mesoporous TiO2 Coated with Au and Pt. J. Nanosci. Nanotechnol. 2020, 20, 5276–5281. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.Y.; Zhang, B.X.; Feng, X.; Yan, X.Y.; Su, Z.; Cheng, S.Z.D.; Yue, K. Controlling the Periodically Ordered Nanostructures in Ceramics: A Macromolecule-Guided Strategy. Macromol. Rapid Commun. 2020, 41, 1900534–1900548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Guo, Y.; Kim, J.; Whitten, A.E.; Wood, K.; Kani, K.; Rowan, A.E.; Henzie, J.; Yamauchi, Y. Mesoporous Metallic Iridium Nanosheets. J. Am. Chem. Soc. 2018, 140, 12434–12441. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Ding, B.; Chang, Z.; Zhang, X.; Yamauchi, Y.; Wu, K.C.W. Confined Self-Assembly in Two-Dimensional Interlayer Space: Monolayered Mesoporous Carbon Nanosheets with In-Plane Orderly Arranged Mesopores and a Highly Graphitized Framework. Angew. Chem. Int. Ed. 2018, 57, 2894–2898. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Young, C.; Kim, J.; You, J.; Yamauchi, Y.; Kuo, S.W. Hollow Microspherical and Microtubular [3 + 3] Carbazole-Based Covalent Organic Frameworks and Their Gas and Energy Storage Applications. ACS Appl. Mater. Interface 2019, 11, 9343–9354. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.M.; Wang, Q.H. Ultralow-dielectric, nanoporous poly (methyl silsesquioxanes) films templated by a self-assembled block copolymer upon solvent annealing. J. Polym. Res. 2019, 26, 5. [Google Scholar] [CrossRef]
- Wang, Z.B.; Qiang, H.W.; Zhang, C.L.; Zhu, Z.H.; Chen, M.; Chen, C.N.; Zhang, D.W. Facile fabrication of hollow polyaniline spheres and its application in supercapacitor. J. Polym. Res. 2018, 25, 129. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.M.; Mohamed, M.G.; Mansoure, T.H.; Yu, H.H.; Chen, T.; Kuo, S.W. Ultrastable tetraphenyl-p-phenylenediamine-based covalent organic frameworks as platforms for high-performance electrochemical supercapacitors. Chem. Commun. 2019, 55, 14890–14893. [Google Scholar] [CrossRef]
- Wu, Y.C.; Lu, Y.S.; Bastakoti, B.P.; Li, Y.; Pramanik, M.; Hossain, M.S.; Yanmaz, E.; Kuo, S.W. Mesoporous TiO2 Thin Film Formed from a Bioinspired Supramolecular Assembly. ChemistrySelect 2016, 1, 4295–4299. [Google Scholar] [CrossRef]
- Tsai, C.C.; Gan, Z.; Kuo, S.W. Using benzoxazine chemistry and bio-based triblock copolymer to prepare functional porous polypeptide capable of efficient dye adsorption. Polym. Chem. 2018, 9, 3684–3693. [Google Scholar] [CrossRef]
- Lu, Y.S.; Bastakoti, B.P.; Pramanik, M.; Malgras, V.; Yamauchi, Y.; Kuo, S.W. Direct Assembly of Mesoporous Silica Functionalized with Polypeptides for Efficient Dye Adsorption. Chem. Eur. J. 2016, 22, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.C.; Peng, D.R.; Baskakoti, B.P.; Pramanik, M.; Malgras, V.; Ahamad, T.; Alshehri, S.M.; Yamauchi, Y.; Kuo, S.W. Co-templating Synthesis of Bimodal Mesoporous Silica for Potential Drug Carrier. ChemistrySelect 2016, 1, 1339–1346. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Langley, P.J.; Hulliger, J. Nanoporous and mesoporous organic structures: New openings for materials research. Chem. Soc. Rev. 1999, 28, 279–291. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Muylaert, I.; Verberckmoes, A.; Decker, J.D.; Voort, P.V.D. Ordered mesoporous phenolic resins: Highly versatile and ultrastable support materials. Adv. Colloid Interface Sci. 2012, 175, 39–51. [Google Scholar] [CrossRef]
- Li, J.G.; Chen, W.C.; Kuo, S.W. Phase behavior of mesoporous silicas templated by the amphiphilic diblock copolymer poly (ethylene-b-ethylene oxide). Microporous Mesoporous Mater. 2012, 163, 34–41. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen Bonding in Polymeric Materials; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Su, W.C.; Tsai, F.C.; Huang, C.F.; Dai, L.; Kuo, S.W. Flexible Epoxy Resins Formed by Blending with the Diblock Copolymer PEO-b-PCL and Using a Hydrogen-Bonding Benzoxazine as the Curing Agent. Polymers 2019, 11, 201. [Google Scholar] [CrossRef] [Green Version]
- Dobrosielska, K.; Wakao, S.; Takano, A.; Matsushita, Y. Nanophase-separated structures of AB block copolymer/C homopolymer blends with complementary hydrogen-bonding interactions. Macromolecules 2008, 41, 7695–7698. [Google Scholar] [CrossRef]
- Kwak, J.; Han, S.H.; Moon, H.C.; Kim, J.K.; Koo, J.; Lee, J.S.; Pryamitsyn, V.; Ganesan, V. Phase behavior of binary blend consisting of asymmetric polystyrene-block-poly (2-vinylpyridine) copolymer and asymmetric deuterated polystyrene-block-poly (4-hydroxystyrene) copolymer. Macromolecules 2015, 48, 1262–1266. [Google Scholar] [CrossRef]
- Miyase, H.; Asai, Y.; Takano, A.; Matsushita, Y. Kaleidoscopic Tiling Patterns with Large Unit Cells from ABC Star-Shaped Terpolymer/Diblock Copolymer Blends with Hydrogen Bonding Interaction. Macromolecules 2017, 50, 979–986. [Google Scholar] [CrossRef]
- Tsou, C.T.; Kuo, S.W. Competing Hydrogen Bonding Interaction Creates Hierarchically Ordered Self-Assembled Structures of PMMA-b-P4VP/PVPh-b-PS Mixtures. Macromolecules 2019, 52, 8374–8383. [Google Scholar] [CrossRef]
- Mao, B.H.; EL-Mahdy, A.F.M.; Kuo, S.W. Bio-inspired multiple complementary hydrogen bonds enhance the miscibility of conjugated polymers blended with polystyrene derivatives. J. Polym. Res. 2019, 26, 208. [Google Scholar] [CrossRef]
- Kwak, J.; Han, S.H.; Moon, H.C.; Kim, J.K. Effect of the degree of hydrogen bonding on asymmetric lamellar microdomains in binary block copolymer blends. Macromolecules 2015, 48, 6347–6352. [Google Scholar] [CrossRef]
- Tseng, T.C.; Kuo, S.W. Hydrogen bonding induces unusual self-assembled structures from mixtures of two miscible disordered diblock copolymers. Eur. Polym. J. 2019, 116, 361–369. [Google Scholar] [CrossRef]
- Tseng, T.C.; Kuo, S.W. Hierarchical Self-Assembled Structures from Diblock Copolymer Mixtures by Competitive Hydrogen Bonding Strength. Molecules 2018, 23, 2242. [Google Scholar] [CrossRef] [Green Version]
- Tseng, T.C.; Kuo, S.W. Hydrogen-Bonding Strength Influences Hierarchical Self-Assembled Structures in Unusual Miscible/Immiscible Diblock Copolymer Blends. Macromolecules 2018, 51, 6451–6459. [Google Scholar] [CrossRef]
- Su, W.C.; Wu, Y.S.; Wang, C.F.; Kuo, S.W. Self-Assembled Structures of Diblock Copolymer/Homopolymer Blends through Multiple Complementary Hydrogen Bonds. Crystals 2018, 8, 330. [Google Scholar] [CrossRef] [Green Version]
- Soler-Illia, G.J.; Crepaldi, E.L.; Grosso, D.; Sanchez, C. Block copolymer-templated mesoporous oxides. Curr. Opin. Colloid Interface Sci. 2003, 8, 109–126. [Google Scholar] [CrossRef]
- Wei, J.; Wang, H.; Deng, Y.; Sun, Z.; Shi, L.; Tu, B.; Luqman, M.; Zhao, D. Solvent evaporation induced aggregating assembly approach to three-dimensional ordered mesoporous silica with ultralarge accessible mesopores. J. Am. Chem. Soc. 2011, 133, 20369–20377. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Lin, Y.D.; Kuo, S.W. From Microphase Separation to Self-Organized Mesoporous Phenolic Resin through Competitive Hydrogen Bonding with Double-Crystalline Diblock Copolymers of Poly (ethylene oxide-b-ε-caprolactone). Macromolecules 2011, 44, 9295–9309. [Google Scholar] [CrossRef]
- Li, J.G.; Chu, W.C.; Jeng, U.; Kuo, S.W. In Situ Monitoring of the Reaction-Induced Self-Assembly of Phenolic Resin Templated by Diblock Copolymers. Macromol. Chem. Phys. 2013, 214, 2115–2123. [Google Scholar] [CrossRef]
- Liu, C.C.; Chu, W.C.; Li, J.G.; Kuo, S.W. Mediated Competitive Hydrogen Bonding Form Mesoporous Phenolic Resins Templated by Poly (ethylene oxide-b-ε-caprolactone-b-l-lactide) Triblock Copolymers. Macromolecules 2014, 47, 6389–6400. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, W.C.; Chiang, S.F.; Li, J.G.; Kuo, S.W. Mesoporous silicas templated by symmetrical multiblock copolymers through evaporation-induced self-assembly. RSC Adv. 2014, 4, 784–793. [Google Scholar] [CrossRef]
- Kosonen, H.; Ruokolainen, J.; Torkkeli, M.; Serimaa, R.; Nyholm, P.; Ikkala, O. Micro- and macrophase separation in phenolic resol resin/PEO-PPO-PEO block copolymer blends: Effect of hydrogen-bonded PEO length. Macromol. Chem. Phys. 2002, 203, 388–392. [Google Scholar] [CrossRef]
- Liang, C.; Dai, S. Synthesis of mesoporous carbon materials via enhanced hydrogen-bonding interaction. J. Am. Chem. Soc. 2016, 128, 5316–5317. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Cheng, L.; Feng, D.; Wu, Z.; Chen, Z.; Wan, Y.; Stein, A.; et al. A family of highly ordered mesoporous polymer resin and carbon structures from organic-organic self-assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Chu, W.C.; Chiang, S.F.; Li, J.G.; Kuo, S.W. Hydrogen bonding-mediated microphase separation during the formation of mesoporous novolac-type phenolic resin templated by the triblock copolymer, PEO-b-PPO-b-PEO. Materials 2013, 6, 5077–5093. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Yu, T.; Wang, Y.; Shi, Y.; Meng, Y.; Gu, D.; Zhang, L.; Huang, Y.; Liu, C.; Wu, X.; et al. Ordered mesoporous silicas and carbons with large accessible pores templated from amphiphilic diblock copolymer poly (ethylene oxide)-b-polystyrene. J. Am. Chem. Soc. 2007, 29, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.; Llewellyn, P.L.; Phan, T.; Bertin, D.; Hornebecq, V. On defining a simple empirical relationship to predict the pore size of mesoporous silicas prepared from PEO-b-PS diblock copolymers. Chem. Mater. 2009, 21, 48–55. [Google Scholar] [CrossRef]
- Hu, D.; Xu, Z.; Zeng, K.; Zheng, S. From self-organized Novolac resins to ordered nanoporous carbons. Macromolecules 2010, 43, 2960–2969. [Google Scholar] [CrossRef]
- Chu, W.C.; Bastakoti, B.P.; Kaneti, Y.V.; Li, J.G.; Alamri, H.R.; Alothman, Z.A.; Yamauchi, Y.; Kuo, S.W. Tailored Design of Bicontinuous Gyroid Mesoporous Carbon and Nitrogen-Doped Carbon from Poly (ethylene oxide-b-caprolactone) Diblock Copolymers. Chem. Eur. J. 2017, 23, 13734–13741. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Ho, Y.F.; Ahmed, M.M.M.; Liang, H.C.; Kuo, S.W. Mesoporous Carbons Templated by PEO-PCL Block Copolymers as Electrode Materials for Supercapacitors. Chem. Eur. J. 2019, 25, 10456–10463. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Liu, T.E.; Kuo, S.W. Direct Synthesis of Nitrogen-Doped Mesoporous Carbons from Triazine-Functionalized Resol for CO2 Uptake and Highly Efficient Removal of Dyes. J. Hazard. Mater. 2020, 391, 122163. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, C.; Gu, D.; Yu, T.; Tu, B.; Zhao, D. Thick wall mesoporous carbons with a large pore structure templated from a weakly hydrophobic PEO–PMMA diblock copolymer. J. Mater. Chem. 2008, 18, 91–97. [Google Scholar] [CrossRef]
- Altukhov, O.; Kuo, S.W. Crystallization ability of poly (lactic acid) block segments in templating poly (ethylene oxide-b-lactic acid) diblock copolymers affects the resulting structures of mesoporous silicas. RSC Adv. 2015, 5, 22625–22637. [Google Scholar] [CrossRef]
- Li, J.G.; Lee, P.Y.; Ahmed, M.M.M.; Mohamed, M.G.; Kuo, S.W. Varying the Hydrogen Bonding Strength in Phenolic/PEO-b-PLA Blends Provides Mesoporous Carbons Having Large Accessible Pores Suitable for Energy Storage. Macromol. Chem. Phys. 2020, 221, 2000040. [Google Scholar] [CrossRef]
- Werner, J.G.; Hoheisel, T.N.; Wiesner, U. Synthesis and characterization of gyroidal mesoporous carbons and carbon monoliths with tunable ultralarge pore size. ACS Nano 2014, 8, 731–743. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, J.; Liu, C.; Gu, D.; Sun, Z.; Wei, J.; Zhang, J.; Tu, B.; Zhao, D. Ultra-large-pore mesoporous carbons templated from poly (ethylene oxide)-b-polystyrene diblock copolymer by adding polystyrene homopolymer as a pore expander. Chem. Mater. 2008, 20, 7281–7286. [Google Scholar] [CrossRef]
- Wei, J.; Deng, Y.; Zhang, J.; Sun, Z.; Tu, B.; Zhao, D. Large-pore ordered mesoporous carbons with tunable structures and pore sizes. Solid State Sci. 2011, 13, 784–792. [Google Scholar] [CrossRef]
- Hung, W.S.; Ahmed, M.M.M.; Mohamed, M.G.; Kuo, S.W. Competing Hydrogen Bonding Produces Mesoporous/Macroporous Carbons Templated by a High-Molecular-Weight Poly (caprolactone–b–ethylene oxide–b–caprolactone) Triblock Copolymer. J. Polym. Res. 2020, 27, 173. [Google Scholar] [CrossRef]
- Kuo, S.W.; Lin, C.L.; Chang, F.C. Phase Behavior and Hydrogen Bonding in Ternary Polymer Blends of Phenolic Resin/Poly (ethylene oxide)/Poly (ε-caprolactone). Macromolecules 2002, 35, 278–285. [Google Scholar] [CrossRef]
- Huang, C.F.; Kuo, S.W.; Lin, F.J.; Huang, W.J.; Wang, C.F.; Chen, W.Y.; Chang, F.C. Influence of PMMA-Chain-End Tethered Polyhedral Oligomeric Silsesquioxanes on the Miscibility and Specific Interaction with Phenolic Blends. Macromolecules 2006, 39, 300–308. [Google Scholar] [CrossRef]
- Huang, C.F.; Kuo, S.W.; Lin, H.C.; Chen, J.K.; Chen, Y.K.; Xu, H.; Chang, F.C. Thermal properties, miscibility and specific interactions in comparison of linear and star poly (methyl methacrylate) blend with phenolic. Polymer 2004, 45, 5913–5921. [Google Scholar] [CrossRef]
- Huang, M.W.; Kuo, S.W.; Wu, H.D.; Chang, F.C.; Fang, S.Y. Miscibility and hydrogen bonding in blends of poly (vinyl acetate) with phenolic resin. Polymer 2002, 43, 2479–2487. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. Miscibility Behavior and Specific Interaction of Phenolic Resin with Poly (acetoxystyrene) Blends. Macromol. Chem. Phys. 2002, 203, 868–878. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, M.; Manna, A.; Krishna, R.; Das, S. Dual Strategic Approach to Prepare Defluorinated Triazole-Embedded Covalent Triazine Frameworks with High Gas Uptake Performance. Chem. Mater. 2019, 31, 3929–3940. [Google Scholar] [CrossRef]
- Mohamed, M.G.; EL-Mahdy, A.F.M.; Ahmed, M.M.M.; Kuo, S.W. Direct Synthesis of Microporous Bicarbazole-Based Covalent Triazine Frameworks for High-Performance Energy Storage and Carbon Dioxide Uptake. ChemPlusChem 2019, 84, 1767–1774. [Google Scholar] [CrossRef]
- Hao, L.; Ning, J.; Luo, B.; Wang, B.; Zhang, Y.; Tang, Z.; Yang, J.; Thomas, A.; Zhi, L. Structural Evolution of 2D Microporous Covalent Triazine-Based Framework toward the Study of High-Performance Supercapacitors. J. Am. Chem. Soc. 2015, 137, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, L.; Lai, G.; Wei, M.; Jiang, X.; Bai, L. Nitrogen-Doped Hierarchically Porous Carbons Derived from Polybenzoxazine for Enhanced Supercapacitor Performance. Nanomaterials 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.G.; EL-Mahdy, A.F.M.; Takashi, Y.; Kuo, S.W. Ultrastable conductive microporous covalent triazine frameworks based on pyrene moieties provide high-performance CO2 uptake and supercapacitance. New J. Chem. 2020. [Google Scholar] [CrossRef]
- Abuzeid, H.R.; EL-Mahdy, A.F.M.; Kuo, S.W. Hydrogen Bonding Induces Dual Porous Types with Microporous and Mesoporous Covalent Organic Frameworks Based on Bicarbazole Units. Microporous Mesoporous Mater. 2020, 300, 110151. [Google Scholar] [CrossRef]
- Yu, L.; Hu, L.; Anasori, B.; Liu, Y.T.; Zhu, Q.; Zhang, P.; Gogotsi, Y.; Xu, B. MXene-Bonded Activated Carbon as a Flexible Electrode for High-Performance Supercapacitors. ACS Energy Lett. 2018, 3, 1597–1603. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent Development in the Production of Activated Carbon Electrodes from Agricultural Waste Biomass for Supercapacitors: A Review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Bhat, B.R.; Sahajwalla, V. Performance of an Activated Carbon Supercapacitor Electrode Synthesised from Waste Compact Discs (CDs). J. Ind. Eng. Chem. 2018, 65, 387–396. [Google Scholar] [CrossRef]
- Wang, D.; Fang, G.; Xue, T.; Ma, J.; Geng, G. A Melt Route for the Synthesis of Activated Carbon Derived from Carton Box for High Performance Symmetric Supercapacitor Applications. J. Power Sources 2016, 307, 401–409. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Q.; Wang, A.; Xu, J.; Wu, S.; Shen, J. Bamboo-like Composites of V2O5/Polyindole and Activated Carbon Cloth as Electrodes for All-Solid-State Flexible Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 3776–3783. [Google Scholar] [CrossRef]
- Ahmed, M.M.M.; Imae, T.; Hill, J.P.; Yamauchi, Y.; Ariga, K.; Shrestha, L.K. Defect-Free Exfoliation of Graphene at Ultra-High Temperature. Coll. Surf. A Physicochem. Eng. Asp. 2018, 538, 127–132. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Dou, G.; Salari, M.; Grinstaff, M.W. Biomass-Based Fuels and Activated Carbon Electrode Materials: An Integrated Approach to Green Energy Systems. ACS Sustain. Chem. Eng. 2017, 5, 3046–3054. [Google Scholar] [CrossRef]
- Zhai, S.; Jiang, W.; Wei, L.; Karahan, H.E.; Yuan, Y.; Ng, A.K.; Chen, Y. All-carbon solid-state yarn supercapacitors from activated carbon and carbon fibers for smart textiles. Mater. Horiz. 2015, 2, 598–605. [Google Scholar] [CrossRef]
- Cao, J.; Jafta, C.J.; Gong, J.; Ran, Q.; Lin, X.; Félix, R.; Wilks, R.G.; Bär, M.; Yuan, J.; Ballauff, M.; et al. Synthesis of Dispersible Mesoporous Nitrogen-Doped Hollow Carbon Nanoplates with Uniform Hexagonal Morphologies for Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 29628–29636. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Ambade, R.B.; Noh, S.H.; Eom, W.; Koh, K.H.; Ambade, S.B.; Lee, W.J.; Kim, S.H.; Han, T.H. Porous Graphene-Carbon Nanotube Scaffolds for Fiber Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 9011–9022. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Kim, Y.J.; Miyawaki, J.; Kim, Y.A.; Yudasaka, M.; Iijima, S.; Kaneko, K. Effect of the Size and Position of Ion-Accessible Nanoholes on the Specific Capacitance of Single-Walled Carbon Nanohorns for Supercapacitor Applications. J. Phys. Chem. C 2015, 119, 2935–2940. [Google Scholar] [CrossRef]
- Stimpfling, T.; Leroux, F. Supercapacitor-Type Behavior of Carbon Composite and Replica Obtained from Hybrid Layered Double Hydroxide Active Container. Chem. Mater. 2010, 22, 974–987. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Parveen, A.S.; Lee, Y.R.; Kim, S.C. Polybenzoxazine originated N-doped mesoporous carbon ropes as an electrode material for high-performance supercapacitors. J. Alloy. Compd. 2018, 750, 384–391. [Google Scholar] [CrossRef]









| Phenolic/LEL | d Spacing (X-ray, nm) | Pore Size (BET, nm) | Pore Size (TEM, nm) | SBET (m2/g) | VTotal (cm3/g) | VMeso (cm3/g) |
|---|---|---|---|---|---|---|
| 70/30 | 51.2 | 30.1 ± 11.3 | 26.5 ± 4.9 | 441.3 | 0.27 | 0.18 |
| 60/40 | 61.6 | 45.6 ± 11.3 | 54 ± 11.6 | 609.7 | 0.48 | 0.23 |
| 50/50 | 54.9 | 44.7 ± 11.4 | 36.8 ± 9.2 | 564.2 | 0.42 | 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.G.; Hung, W.-S.; EL-Mahdy, A.F.M.; Ahmed, M.M.M.; Dai, L.; Chen, T.; Kuo, S.-W. High-Molecular-Weight PLA-b-PEO-b-PLA Triblock Copolymer Templated Large Mesoporous Carbons for Supercapacitors and CO2 Capture. Polymers 2020, 12, 1193. https://doi.org/10.3390/polym12051193
Mohamed MG, Hung W-S, EL-Mahdy AFM, Ahmed MMM, Dai L, Chen T, Kuo S-W. High-Molecular-Weight PLA-b-PEO-b-PLA Triblock Copolymer Templated Large Mesoporous Carbons for Supercapacitors and CO2 Capture. Polymers. 2020; 12(5):1193. https://doi.org/10.3390/polym12051193
Chicago/Turabian StyleMohamed, Mohamed Gamal, Wei-Shih Hung, Ahmed F. M. EL-Mahdy, Mahmoud M. M. Ahmed, Lizong Dai, Tao Chen, and Shiao-Wei Kuo. 2020. "High-Molecular-Weight PLA-b-PEO-b-PLA Triblock Copolymer Templated Large Mesoporous Carbons for Supercapacitors and CO2 Capture" Polymers 12, no. 5: 1193. https://doi.org/10.3390/polym12051193