Chitosan-Sulfated Titania Composite Membranes with Potential Applications in Fuel Cell: Influence of Cross-Linker Nature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Membranes
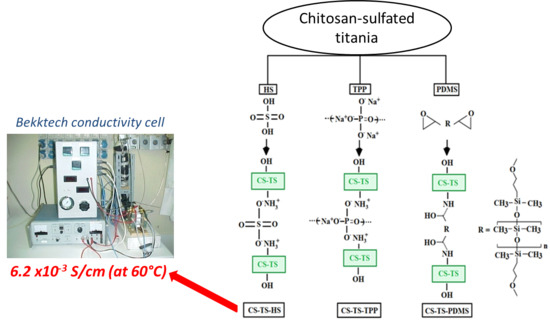
2.2.1. Preparation of Chitosan-Sulfated Titania Composite Membranes (CS-TS)
2.2.2. Membrane Cross-Linking by Sulfuric Acid (HS) and by Pentasodium Tripolyphosphate (TPP)
2.2.3. Cross-Linking by Bis(glycidyloxypropyl)-Terminated Polydimethylsiloxane (PDMS)
2.3. Materials Characterization
3. Results and Discussions
3.1. Membrane Preparation
3.2. FTIR Characterization
3.3. SEM Characterization
3.4. Thermogravimetric Analysis
3.5. Mechanical Properties
3.6. Water Uptake Capacity of Composite Membranes
3.7. Chemical Stability of Composite Membranes
3.8. Broadband Dielectric Spectroscopy
3.8.1. Overall Dielectric Behavior of Dry Membranes
3.8.2. Influence of Water Absorption on the Protonic Conductivity of Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, M.; Song, Y.; Hu, W.; Li, X.; Jiang, Z.; Guiver, M.D.; Liu, B. SPAEK-based binary blends and ternary composites as proton exchange membranes for DMFCs. J. Membr. Sci. 2012, 415, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Devrim, Y.; Erkan, S.; Bac, N.; Eroglu, I. Preparation and characterization of sulfonated polysulfone/titanium dioxide composite membranes for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2009, 34, 3467–3475. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Panero, S.; Navarra, M.A. Polymer Electrolyte Membranes Based on Nafion and a Superacidic Inorganic Additive for Fuel Cell Applications. Polymers 2019, 11, 914. [Google Scholar] [CrossRef] [Green Version]
- Di Noto, V.; Piga, M.; Pace, G.; Negro, E.; Lavina, S. Dielectric Relaxations and Conductivity Mechanism of Nafion: Studies Based on Broadband Dielectric Spectroscopy. ECS Trans. 2008, 16, 1183–1193. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Chitosan and alginate types of bio-membrane in fuel cell application: An overview. J. Power Sources 2015, 289, 71–80. [Google Scholar] [CrossRef]
- Saccà, A.; Carbone, A.; Gatto, I.; Pedicini, R.; Freni, A.; Patti, A.; Passalacqua, E. Composites Nafion-titania membranes for Polymer Electrolyte Fuel Cell (PEFC) applications at low relative humidity levels: Chemical physical properties and electrochemical performance. Polym. Test. 2016, 56, 10–18. [Google Scholar] [CrossRef]
- Kreuer, K.D. Hydrocarbon membranes. In Handbook of Fuel Cells: Fundamentals, Technology and Applications. In Fuel Cell Technology and Applications; Vielstich, W., Lamm, A., Gasteiger, H., Eds.; John Wiley & Sons Ltd.: London, UK, 2003; Volume 3, pp. 420–435. [Google Scholar]
- Divya, K.; Rana, D.; Alwarappan, S.; Saraswathi, M.S.S.A.; Nagendran, A. Investigating the usefulness of chitosan based proton exchange membranes tailored with exfoliated molybdenum disulfide nanosheets for clean energy applications. Carbohydr. Polym. 2019, 208, 504–512. [Google Scholar] [CrossRef]
- Wang, W.; Shan, B.; Zhu, L.; Xie, C.; Liu, C.; Cui, F. Anatase titania coated CNTs and sodium lignin sulfonate doped chitosan proton exchange membrane for DMFC application. Carbohydr. Polym. 2018, 187, 35–42. [Google Scholar] [CrossRef]
- Santamaria, M.; Pecoraro, C.; Di Franco, F.; Di Quarto, F.; Gatto, I.; Saccà, A. Improvement in the performance of low temperature H2–O2 fuel cell with chitosan–phosphotungstic acid composite membranes. Int. J. Hydrog. Energy 2016, 41, 5389–5395. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wu, H.; Xiao, L.; Jiang, Z. Fabrication and performances of solid superacid embedded chitosan hybrid membranes for direct methanol fuel cell. J. Power Sources 2010, 195, 2526–2533. [Google Scholar] [CrossRef]
- Wang, J.; Gong, C.; Wen, S.; Liu, H.; Qin, C.; Xiong, C.; Dong, L. Proton exchange membrane based on chitosan and solvent-free carbon nanotube fluids for fuel cells applications. Carbohydr. Polym. 2018, 186, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Zheng, Y.P.; Wang, T.H. Sulfated mesoporous Au/TiO2 spheres as a highly active and stable solid acid catalyst. J. Mater. Chem. 2012, 22, 13216. [Google Scholar] [CrossRef]
- Ayyaru, S.; Dharmalingam, S. Improved performance of microbial fuel cells using sulfonated polyether ether ketone (SPEEK) TiO2-SO3H nanocomposite membrane. RSC Adv. 2013, 3, 25243–25251. [Google Scholar] [CrossRef]
- Cui, Z.; Xiang, Y.; Si, J.; Yang, M.; Zhang, Q.; Zhang, T. Ionic interactions between sulfuric acid and chitosan membranes. Carbohydr. Polym. 2008, 73, 111–116. [Google Scholar] [CrossRef]
- Gierszewska-Drużyńska, M.; Ostrowska-Czubenko, J. Influence of Crosslinking Process Conditions on Molecular and Supermolecular Structure of Chitosan Hydrogel Membrane. Prog. Chem. Appl. Chitin Deriv. 2011, 16, 15–22. [Google Scholar]
- Enescu, D.; Hamciuc, V.; Pricop, L.; Hamaide, T.; Harabagiu, V.; Simionescu, B.C. Polydimethylsiloxane-modified chitosan I. Synthesis and structural characterisation of graft and crosslinked copolymers. J. Polym. Res. 2008, 16, 73–80. [Google Scholar] [CrossRef]
- Saccà, A.; Carbone, A.; Gatto, I.; Pedicini, R.; Passalacqua, E. Synthesized Yttria Stabilised Zirconia as filler in Proton Exchange Membranes (PEMs) with enhanced stability. Polym. Test. 2018, 65, 322–330. [Google Scholar] [CrossRef]
- Angjeli, K.; Nicotera, I.; Baikousi, M.; Enotiadis, A.; Gournis, D.; Saccà, A.; Passalacqua, E.; Carbone, A. Investigation of layered double hydroxide (LDH) Nafion-based nanocomposite membranes for high temperature PEFCs. Energy Convers. Manag. 2015, 96, 39–46. [Google Scholar] [CrossRef]
- Enescu, D.; Hamciuc, V.; Ardeleanu, R.; Cristea, M.; Ioanid, A.; Harabagiu, V.; Simionescu, B.C. Polydimethylsiloxane modified chitosan. Part III: Preparation and characterization of hybrid membranes. Carbohydr. Polym. 2009, 76, 268–278. [Google Scholar] [CrossRef]
- Kumar, K.S.; Rajendran, S.; Prabhu, M.R. A Study of influence on sulfonated TiO2-Poly (Vinylidene fluoride-co-hexafluoropropylene) nano composite membranes for PEM Fuel cell application. Appl. Surf. Sci. 2017, 418, 64–71. [Google Scholar] [CrossRef]
- Humelnicu, A.-C.; Cojocaru, C.; Dorneanu, P.P.; Samoila, P.; Harabagiu, V. Novel chitosan-functionalized samarium-doped cobalt ferrite for adsorptive removal of anionic dye from aqueous solutions. Comptes Rendus Chim. 2017, 20, 1026–1036. [Google Scholar] [CrossRef]
- Das, G.; Kim, C.Y.; Kang, D.H.; Kim, B.H.; Yoon, H.H. Quaternized Polysulfone Cross-Linked N,N-Dimethyl Chitosan-Based Anion-Conducting Membranes. Polymers 2019, 11, 512. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Li, J.; Jiang, Z.; Lu, L.; Chen, X. Chitosan/TiO 2 nanocomposite pervaporation membranes for ethanol dehydration. Chem. Eng. Sci. 2009, 64, 3130–3137. [Google Scholar] [CrossRef]
- Ngah, W.W.; Fatinathan, S.; Yosop, N. Isotherm and kinetic studies on the adsorption of humic acid onto chitosan-H2SO4 beads. Desalination 2011, 272, 293–300. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Alam El-Din, H.M.; Elberry, M.H.; Allam, N.G.; Hasanin, M.T.M.; Abdellah, A.M. Synthesis, characterization and cytotoxic evaluation of chitosan nanoparticles: In vitro liver cancer model. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 35008. [Google Scholar] [CrossRef]
- Simionescu, C.I.; Rusa, M.; David, G.; Pinteala, M.; Harabagiu, V.; Simionescu, B.C. Block and graft copolymers with polysiloxane and poly(N-acyliminoethylene) sequences. Angew. Chernie Makromol. 1997, 253, 139–149. [Google Scholar] [CrossRef]
- Hayward, R.C.; Chmelka, B.F.; Kramer, E.J. Template Cross-Linking Effects on Morphologies of Swellable Block Copolymer and Mesostructured Silica Thin Films. Macromolecules 2005, 38, 7768–7783. [Google Scholar] [CrossRef] [Green Version]
- Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H. Thermal stability of magnetic nanoparticles coated by blends of modified chitosan and poly(quaternary ammonium) salt. J. Therm. Anal. Calorim. 2014, 119, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Pati, F.; Adhikari, B.; Dhara, S. Development of chitosan – tripolyphosphate fibers through pH dependent ionotropic gelation. Carbohydr. Res. 2011, 346, 2582–2588. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Garofalo, M.L.; Lu, Z. Water Management in A PEMFC: Water Transport Mechanism and Material Degradation in Gas Diffusion Layers. Fuel Cells 2011, 11, 814–823. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Rodriguez-Hernandez, A.; Velázquez, G. Modelling the effect of temperature on the water sorption isotherms of chitosan films. Food Sci. Technol. 2016, 37, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Escorihuela, J.; Garcia-Bernabe, A.; Montero, A.; Sahuquillo, Ó.; Giménez, E.; Compañ, V. Ionic Liquid Composite Polybenzimidazol Membranes for High Temperature PEMFC Applications. Polymers 2019, 11, 732. [Google Scholar] [CrossRef] [Green Version]
- Ramly, N.N.; Aini, N.A.; Sahli, N.; Aminuddin, S.F.; Yahya, M.Z.A.; Ali, A.M.M. Dielectric behavior of UV-crosslinked sulfonated poly (ether ether ketone) with methyl cellulose (SPEEK-MC) as proton exchange membrane. Int. J. Hydrog. Energy 2017, 42, 9284–9292. [Google Scholar] [CrossRef]
- Bronnikov, S.; Podshivalov, A.; Kostromin, S.; Asandulesa, M.; Cozan, V. Electrical conductivity of polyazomethine/fullerene C60 nanocomposites. Phys. Lett. A 2017, 381, 796–800. [Google Scholar] [CrossRef]
- Gu, H.; England, D.; Yan, F.; Texter, J. New high charge density polymers for printable electronics, sensors, batteries, and fuel cells. In Proceedings of the 2008 2nd IEEE International Nanoelectronics Conference, Shanghai, China, 24–27 March 2008; pp. 863–868. [Google Scholar]
- Pochard, I.; Vall, M.; Eriksson, J.; Farineau, C.; Cheung, O.; Frykstrand, S.; Welch, K.; Strømme, M. Amine-functionalised mesoporous magnesium carbonate: Dielectric spectroscopy studies of interactions with water and stability. Mater. Chem. Phys. 2018, 216, 332–338. [Google Scholar] [CrossRef]
- Samet, M.; Levchenko, V.; Boiteux, G.; Seytre, G.; Kallel, A.; Serghei, A. Electrode polarization vs. Maxwell-Wagner-Sillars interfacial polarization in dielectric spectra of materials: Characteristic frequencies and scaling laws. J. Chem. Phys. 2015, 142, 194703. [Google Scholar] [CrossRef]
- Asandulesa, M.; Musteata, V.E.; Bele, A.; Dascalu, M.; Bronnikov, S.; Racles, C. Molecular dynamics of polysiloxane polar-nonpolar co-networks and blends studied by dielectric relaxation spectroscopy. Polym. 2018, 149, 73–84. [Google Scholar] [CrossRef]
- Zhang, S.; Runt, J. Segmental Dynamics and Ionic Conduction in Poly(vinyl methyl ether)−Lithium Perchlorate Complexes. J. Phys. Chem. B 2004, 108, 6295–6302. [Google Scholar] [CrossRef]
- Ali, A.; Mohamed, N.; Arof, A.K. Polyethylene oxide (PEO)–ammonium sulfate ((NH4)2SO4) complexes and electrochemical cell performance. J. Power Sources 1998, 74, 135–141. [Google Scholar] [CrossRef]
- Wang, J.; Bai, H.; Zhang, H.; Zhao, L.; Chen, H.; Li, Y. Anhydrous proton exchange membrane of sulfonated poly(ether ether ketone) enabled by polydopamine-modified silica nanoparticles. Electrochim. Acta 2015, 152, 443–455. [Google Scholar] [CrossRef]
- Fischer, S.A.; Dunlap, B.; Gunlycke, D. Proton transport through hydrated chitosan-based polymer membranes under electric fields. J. Polym. Sci. Part B Polym. Phys. 2017, 50, 9–1109. [Google Scholar] [CrossRef]
- Saccà, A.; Carbone, A.; Pedicini, R.; Portale, G.; D’Ilario, L.; Longo, A.; Martorana, A.; Passalacqua, E. Structural and electrochemical investigation on re-cast Nafion membranes for polymer electrolyte fuel cells (PEFCs) application. J. Membr. Sci. 2006, 278, 105–113. [Google Scholar] [CrossRef]
- Saccà, A.; Gatto, I.; Carbone, A.; Pedicini, R.; Maisano, S.; Stassi, A.; Passalacqua, E. Influence of doping level in Yttria-Stabilised-Zirconia (YSZ) based-fillers as degradation inhibitors for proton exchange membranes fuel cells (PEMFCs) in drastic conditions. Int. J. Hydrog. Energy 2019, 44, 31445–31457. [Google Scholar] [CrossRef]











| Membrane | Mechanical Properties | Water Uptake (%) (24 h) | Weight Loss (%) in Fenton Reagent | |||||
|---|---|---|---|---|---|---|---|---|
| Tensile Strain (%) | Tensile Stress (MPa) | Young’s Modulus 2 (GPa) | 25 °C | 60 °C | 80 °C | 1 h | 24 h | |
| CS-TS | 10.6 | 24.9 | 1.54 | dissolution | dissolution | |||
| CS-TS-HS | 11.8 | 40.2 | 1.87 | 184 | 172 | 163 | 10 | 15 |
| CS-TS-TPP | - 1 | 15.3 | 1.46 | 170 | 121 | 135 | 8 | 22 |
| CS-TS-PDMS | 39.7 | 50.9 | 1.01 | 118 | 88 | 92 | 9 | 30 |
| Membrane | Conductivity, σ (S/cm) at a Frequency of 1 Hz for Dry Membranes | Conductivity, σ (S/cm) at Low and High Frequencies for Hydrated Membranes | |||||
|---|---|---|---|---|---|---|---|
| 25 °C | 60 °C | 100 °C | f = 1 Hz | f = 106 Hz | |||
| 25 °C | 60 °C | 25 °C | 60 °C | ||||
| CS-TS | 6.6 × 10−12 | 5.2 × 10−11 | 3.3 × 10−9 | - | - | - | - |
| CS-TS-HS | 2.2 × 10−12 | 1.7 × 10−11 | 3.9 × 10−10 | 2.5 × 10−6 | 8.1 × 10−6 | 2.1 × 10−3 | 1.1 × 10−3 |
| CS-TS-TPP | 1.8 × 10−12 | 1.2 × 10−11 | 7.8 × 10−11 | 5.7 × 10−8 | 5.7 × 10−8 | 5.7 × 10−5 | 4.5 × 10−5 |
| CS-TS-PDMS | 2.6 × 10−12 | 1.1 × 10−11 | 7.4 × 10−11 | 7.0 × 10−8 | 1.9 × 10−7 | 1.4 × 10−5 | 3.1 × 10−5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humelnicu, A.-C.; Samoila, P.; Asandulesa, M.; Cojocaru, C.; Bele, A.; Marinoiu, A.T.; Sacca, A.; Harabagiu, V. Chitosan-Sulfated Titania Composite Membranes with Potential Applications in Fuel Cell: Influence of Cross-Linker Nature. Polymers 2020, 12, 1125. https://doi.org/10.3390/polym12051125
Humelnicu A-C, Samoila P, Asandulesa M, Cojocaru C, Bele A, Marinoiu AT, Sacca A, Harabagiu V. Chitosan-Sulfated Titania Composite Membranes with Potential Applications in Fuel Cell: Influence of Cross-Linker Nature. Polymers. 2020; 12(5):1125. https://doi.org/10.3390/polym12051125
Chicago/Turabian StyleHumelnicu, Andra-Cristina, Petrisor Samoila, Mihai Asandulesa, Corneliu Cojocaru, Adrian Bele, Adriana T. Marinoiu, Ada Sacca, and Valeria Harabagiu. 2020. "Chitosan-Sulfated Titania Composite Membranes with Potential Applications in Fuel Cell: Influence of Cross-Linker Nature" Polymers 12, no. 5: 1125. https://doi.org/10.3390/polym12051125