Improved Process to Obtain Nanofibrillated Cellulose (CNF) Reinforced Starch Films with Upgraded Mechanical Properties and Barrier Character
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
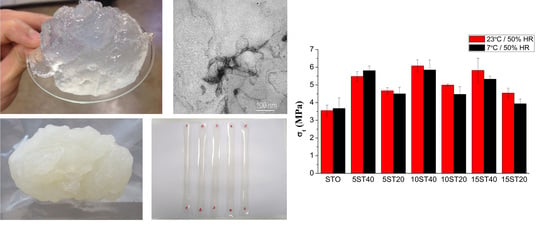
3.1. Processing of the Composite Films
3.2. Water Uptake Behaviour (W.U.)
3.3. Mechanical Characterization
3.4. Hydrolytic Susceptibility in Enzymatic Media
3.5. Oxygen and Water Permeability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Borges, A.C.; Eyholzer, C.; Duc, F.; Bourban, P.-E.; Tingaut, P.; Zimmermann, T.; Pioletti, D.P.; Månson, J.-A.E. Nanofibrillated cellulose composite hydrogel for the replacement of the nucleus pulposus. Acta Biomater. 2011, 7, 3412–3421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarrés, Q.; Saguer, E.; Pèlach, M.A.; Alcalà, M.; Delgado-Aguilar, M.; Mutjé, P. The feasibility of incorporating cellulose micro/nanofibers in papermaking processes: The relevance of enzymatic hydrolysis. Cellulose 2016, 23, 1433–1445. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; González, I.; Tarrés, Q.; Pèlach, M.À.; Alcalà, M.; Mutjé, P. The key role of lignin in the production of low-cost lignocellulosic nanofibres for papermaking applications. Ind. Crops Prod. 2016, 86, 295–300. [Google Scholar] [CrossRef]
- Lay, M.; Méndez, J.A.; Delgado-Aguilar, M.; Bun, K.N.; Vilaseca, F. Strong and electrically conductive nanopaper from cellulose nanofibers and polypyrrole. Carbohydr. Polym. 2016, 152, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Lizundia, E.; Delgado-Aguilar, M.; Mutjé, P.; Fernández, E.; Robles-Hernandez, B.; de la Fuente, M.R.; Vilas, J.L.; León, L.M. Cu-coated cellulose nanopaper for green and low-cost electronics. Cellulose 2016, 23, 1997–2010. [Google Scholar] [CrossRef]
- Yan, J.; Hu, J.; Yang, R.; Zhang, Z.; Zhao, W. Innovative Nanofibrillated Cellulose from Rice Straw as Dietary Fiber for Enhanced Health Benefits Prepared by a Green and Scale Production Method. ACS Sustain. Chem. Eng. 2018, 6, 3481–3492. [Google Scholar] [CrossRef]
- Dimic-Misic, K.; Gane, P.A.C.; Paltakari, J. Micro and nanofibrillated cellulose as a rheology modifier additive in CMC-containing pigment-coating formulations. Ind. Eng. Chem. Res. 2013, 52, 16066–16083. [Google Scholar] [CrossRef]
- Delgado-aguilar, M.; González, I.; Jiménez, A.M.; Tarrés, Q.; Quintana, G.; Mutjé, P. Cellulose Nanofibres Modified with Alkyl Ketene For Oil Absorbent Aerogels. Cellul. Chem. Technol. 2016, 50, 369–375. [Google Scholar]
- Dong, H.; Snyder, J.F.; Tran, D.T.; Leadore, J.L. Hydrogel, aerogel and film of cellulose nanofibrils functionalized with silver nanoparticles. Carbohydr. Polym. 2013, 95, 760–767. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M.; Ankerfors, M.; Lindström, T.; Nordqvist, D.; Mattozzi, A.; Hedenqvist, M.S. Enhanced film forming and film properties of amylopectin using micro-fibrillated cellulose. Carbohydr. Polym. 2007, 68, 718–727. [Google Scholar] [CrossRef]
- Karimi, S.; Tahir, P.; Dufresne, A.; Karimi, A.; Abdulkhani, A. A comparative study on characteristics of nanocellulose reinforced thermoplastic starch biofilms prepared with different techniques. Nord. Pulp Pap. Res. J. 2014, 29, 41–45. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Bher, A.; Unalan, I.U.; Auras, R.; Rubino, M.; Schvezov, C.E. Toughening of poly(lactic acid) and thermoplastic cassava starch reactive blends using graphene nanoplatelets. Polymers 2018, 10, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhadi-Khouzani, M.; Schütz, C.; Durak, G.M.; Fornell, J.; Sort, J.; Salazar-Alvarez, G.; Bergström, L.; Gebauer, D. A CaCO3/nanocellulose-based bioinspired nacre-like material. J. Mater. Chem. A 2017, 5, 16128–16133. [Google Scholar] [CrossRef] [Green Version]
- Olsson, R.T.; Azizi Samir, M.A.S.; Salazar-Alvarez, G.; Belova, L.; Ström, V.; Berglund, L.A.; Ikkala, O.; Nogués, J.; Gedde, U.W. Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat. Nanotechnol. 2010, 5, 584–588. [Google Scholar] [CrossRef]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Kalia, S.; Boufi, S.; Celli, A.; Kango, S. Nanofibrillated cellulose: Surface modification and potential applications. Colloid Polym. Sci. 2013, 292, 5–31. [Google Scholar] [CrossRef]
- Lu, J.; Wang, T.; Drzal, L.T. Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos. Part A Appl. Sci. Manuf. 2008, 39, 738–746. [Google Scholar] [CrossRef]
- Guimarães, M.; Botaro, V.R.; Novack, K.M.; Teixeira, F.G.; Tonoli, G.H.D. Starch/PVA-based nanocomposites reinforced with bamboo nanofibrils. Ind. Crops Prod. 2015, 70, 72–83. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Avena-Bustillos, R.J.; Filho, G.C.; Munford, M.L.; Wood, D.; McHugh, T.H. Nanocellulose Reinforced Chitosan Composite Films as Affected by Nanofiller Loading and Plasticizer Content. J. Food Sci. 2010, 75, N1–N7. [Google Scholar] [CrossRef] [PubMed]
- Frost, K.; Barthes, J.; Kaminski, D.; Lascaris, E.; Niere, J.; Shanks, R. Thermoplastic starch-silica-polyvinyl alcohol composites by reactive extrusion. Carbohydr. Polym. 2011, 84, 343–350. [Google Scholar] [CrossRef]
- Ashori, A.; Babaee, M.; Jonoobi, M.; Hamzeh, Y. Solvent-free acetylation of cellulose nanofibers for improving compatibility and dispersion. Carbohydr. Polym. 2014, 102, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Savadekar, N.R.; Mhaske, S.T. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr. Polym. 2012, 89, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Basiak, E.; Lenart, A.; Debeaufort, F. How glycerol and water contents affect the structural and functional properties of starch-based edible films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hietala, M.; Mathew, A.P.; Oksman, K. Bionanocomposites of thermoplastic starch and cellulose nanofibers manufactured using twin-screw extrusion. Eur. Polym. J. 2013, 49, 950–956. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Lopez-Rubio, A.; Lagaron, J.M. Optimization of the dispersion of unmodified bacterial cellulose nanowhiskers into polylactide via melt compounding to significantly enhance barrier and mechanical properties. Biomacromolecules 2012, 13, 3887–3899. [Google Scholar] [CrossRef]
- Espert, A.; Vilaplana, F.; Karlsson, S. Comparison of water absorption in natural cellulosic fibres from wood and one-year crops in polypropylene composites and its influence on their mechanical properties. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1267–1276. [Google Scholar] [CrossRef]
- Ghanbari, A.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Mashkour, M. Preparation and characterization of thermoplastic starch and cellulose nanofibers as green nanocomposites: Extrusion processing. Int. J. Biol. Macromol. 2018, 112, 442–447. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.F.; Antunes, F.E.; Romano, A.; Miguel, M.G.; Lindman, B. On the role of hydrophobic interactions in cellulose dissolution and regeneration: Colloidal aggregates and molecular solutions. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 257–263. [Google Scholar] [CrossRef]
- Alves, L.; Ferraz, E.; Gamelas, J.A.F. Composites of nanofibrillated cellulose with clay minerals: A review. Adv. Colloid Interface Sci. 2019, 272, 101994. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; González, I.; Oliver-Ortega, H.; Tarrès, Q.; Delgado-Aguilar, M.; Mutjé, P. Reducing the amount of catalyst in TEMPO-oxidized cellulose nanofibers: Effect on properties and cost. Polymers 2017, 9, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez, J.A.; Vilaseca, F.; Pèlach, M.A.; López, J.P.; Barberà, L.; Turon, X.; Gironès, J.; Mutjé, P. Evaluation of the reinforcing effect of ground wood pulp in the preparation of polypropylene-based composites coupled with maleic anhydride grafted polypropylene. J. Appl. Polym. Sci. 2007, 105, 3588–3596. [Google Scholar] [CrossRef]
- Franco-Marquès, E.; Méndez, J.A.; Pèlach, M.A.; Vilaseca, F.; Bayer, J.; Mutjé, P. Influence of coupling agents in the preparation of polypropylene composites reinforced with recycled fibers. Chem. Eng. J. 2011, 166, 1170–1178. [Google Scholar] [CrossRef]
- Reis, R.L.; Cunha, A.M. Starch and Starch Based Thermoplastics. In Encyclopedia of Materials Science and Technology, 3rd ed.; Jürgen Buschow, K.H., Cahn, R.W., Eds.; Elsevier: Oxford, UK, 2001; Volume 1, pp. 8810–8816. [Google Scholar]





| Formulation | Oxidation * (mmol) | Starch (g) | Gly-CNFBEP ϕ (g) | CNFBEP++ (g) | Glycerol (g) | H2O (g) | CNFBEP‡ (%) |
|---|---|---|---|---|---|---|---|
| ST0 (ref.) | --- | 60 | --- | --- | 40 | 12 | --- |
| 5ST20 | 5 | 60 | 20 | 0.2 | 20 | 12 | 0.18 |
| 5ST40 | 5 | 60 | 40 | 0.4 | --- | 12 | 0.36 |
| 10ST20 | 10 | 60 | 20 | 0.2 | 20 | 12 | 0.18 |
| 10ST40 | 10 | 60 | 40 | 0.4 | --- | 12 | 0.36 |
| 15ST20 | 15 | 60 | 20 | 0.2 | 20 | 12 | 0.18 |
| 15ST40 | 15 | 60 | 40 | 0.4 | --- | 12 | 0.36 |
| Sample | WVP·1013 (Kg·m·Pa−1 s−1·m−2) | OP·1019·(m3·m·Pa−1·s−1·m−2) |
|---|---|---|
| STO-1 | 6.19 (0.72) a | 1.11 (0.06) a |
| STO-2 | 5.90 (0.90) a | 1.23 (0.04) a |
| 10ST20-1 | 6.63 (0.85) a | 1.35 (0.13) a |
| 10ST20-2 | 5.65 (0.28) a | 1.16 (0.15) a |
| 10ST40-1 | 2.96 (0.71) b | 0.54 (0.01) b |
| 10ST40-2 | 2.72 (0.85) b | 0.60 (0.06) b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granda, L.A.; Oliver-Ortega, H.; Fabra, M.J.; Tarrés, Q.; Pèlach, M.À.; Lagarón, J.M.; Méndez, J.A. Improved Process to Obtain Nanofibrillated Cellulose (CNF) Reinforced Starch Films with Upgraded Mechanical Properties and Barrier Character. Polymers 2020, 12, 1071. https://doi.org/10.3390/polym12051071
Granda LA, Oliver-Ortega H, Fabra MJ, Tarrés Q, Pèlach MÀ, Lagarón JM, Méndez JA. Improved Process to Obtain Nanofibrillated Cellulose (CNF) Reinforced Starch Films with Upgraded Mechanical Properties and Barrier Character. Polymers. 2020; 12(5):1071. https://doi.org/10.3390/polym12051071
Chicago/Turabian StyleGranda, Luis Angel, Helena Oliver-Ortega, Maria José Fabra, Quim Tarrés, Maria Àngels Pèlach, José Maria Lagarón, and José Alberto Méndez. 2020. "Improved Process to Obtain Nanofibrillated Cellulose (CNF) Reinforced Starch Films with Upgraded Mechanical Properties and Barrier Character" Polymers 12, no. 5: 1071. https://doi.org/10.3390/polym12051071