3. Results and Discussion
Polyurethanes (PURs) were obtained through the prepolymer method detailed in our previous work [
7,
9]. The diagram of PURs synthesis is presented in
Figure 1.
Three series of polyurethanes, with different amount of PCLt, were obtained. Polyurethanes in each series differed in the construction of soft segments. Hard segments were synthetized with H12MDI and 1,4-BD in each case. The molar ratio of NCO:OH in prepolymer was always 2:1, whereas the total ratio in PUR was 1:1.
The chemical composition of synthetized PURs is presented in
Table 1. PUR series are named according to percentage of oligomerol in soft segments (wt %) of the used R,S-PHB/PCL
t system.
Percentage content of side CH
3 (from R,S-PHB) and side C
2H
5 (from PCL
t) was estimated according to [
10] and is given in
Table 1. As C
2H
5 is attached to the carbon at the branching point of PCL
t, an increase in its amount in the PUR structure indicates an increase in the degree of branching. Cross-linking and branching density of the polymer network is also expressed through the calculated molecular weight of chains between the branching/cross-linking point (
Mc) [
11], and is calculated according to [
10].
Considering starting substrates used for the synthesis of polyurethanes, it can be assumed that their properties will be influenced by the type and the amount of polyols used, and this will also affect the structure of the polymer: Chain length between branching/cross-linking nodes, and the number of short methyl and ethyl chains, which are a steric hindrance [
10].
An increase in the amount of PCLt in soft segments decreases the chain length between branch nodes, i.e., an increase in Mc. At the same time the number of ethyl side groups increases. Whereas, when the amount of R,S-PHB increases, the number of side methyl groups increases simultaneously.
The synthetized polyurethanes were almost transparent, and those based on R,S-PHB obtained from iodoethanole were slightly yellow (
Table 2). Their density was about 1.1 g/cm
−3, independently of the amount of the added triol. Polyurethanes, obtained with 5 wt % of PCL
t, were soluble in DMF, which indicated that they were branched; whereas PURs based on the higher amount of PCL
t swelled in DMF, due to the formation of the cross-linked structure. However, it did not affect the oil sorption of PURs, which stays at the same level for all samples studied.
The chemical structure of the synthesized PURs was confirmed via FTIR spectroscopy (
Figure S1, Supplementary Materials). The characteristic absorption bands which appeared in the FTIR spectra and proved the urethane linkage formation were: A broad absorption band centered at about 3350 cm
−1, which corresponded to –NH groups (–NH stretching vibrations), the peak corresponding to C=O stretching vibrations observed around 1720 cm
−1, and peaks of an amide II band and an amide III band around 1520 and 1240 cm
−1, respectively (
Table S1, Supplementary Materials). On FTIR spectra of PURs, the band corresponding to –NCO (2270 cm
−1) disappeared. Generally, individual spectra of the all polyurethanes were very similar (
Figure S1, Supplementary Materials).
The main differences were observed in the multiple band in the range 1040 cm
−1 to 1245 cm
−1 assigned to stretching C–O bonds in soft segments [
12] (
Figure 2). Shifting these peaks in this range, after adding or increasing the amount of R,S-PHB, indicated that this polyol was successfully incorporated into the PURs structure (
Figure S2, Supplementary Materials).
Introducing an amorphous R,S-PHB with a lower distance between carbonyl groups in the chain than PCL into soft segments increased possible interaction between urethane and carbonyl groups. Such an increase in hydrogen bond formation after introducing a substrate with a larger number of hydrogen bonding sites was already observed [
13]. In consequence, more hydrogen bonded –N–H groups were formed and the band of their stretching vibration (around 3350 cm
−1) shifted to a lower wavenumber (
Table S1, Supplementary Materials).
The intensity of the C–H stretching vibration around 2920 cm
−1 (
Figure S1, Supplementary Materials) was used as the standard against which the intensity of the urethane carbonyl bands at 1720 cm
−1 was compared. As the length of the repeating mer in R,S-PHB was shorter than in PCL, the frequency of arising urethane groups and the amount of carbonyl ester groups were greater in polyurethane structure after R,S-PHB using for the synthesis of soft segments. This caused the intensity of the carbonyl of PUR x/5 to increase with increasing amounts of R,S-PHB. This indicated the presence of R,S-PHB on the polyurethane surface. However, after using a higher amount of PCL
t for PUR x/15 and PUR x/20 building, the changes in peak intensity were not so straightforward. The reason was probably the inhibition of chain mobility after increasing the number of crosslinks.
Crystallinity of the synthetized PURs was low. Only PURs based on 5 wt % of PCL
t in soft segments had the pronounced melting peak with significant melting enthalpy (
Figure 3). Increasing R,S-PHB in the soft segments structure of PURs with the higher amount of PCL
t increased discreetly melting enthalpy. Despite the amorphous nature of R,S-PHB, its presence in the polymer network facilitated PCL chains moving and forming into crystalline forms. After the ordering of PCL chains, they were stabilized by hydrogen bonds formed due to R,S-PHB presence. Next, these ordered chains were formed into crystalline forms, which was the reason the melting enthalpy increased for PURs with intermate quantities of R,S-PHB present. However, if the amount of R,S-PHB was even greater, its amorphous nature caused the soft segment to become more amorphous. It could be seen that the
Tm also slightly increased (
Table 3).
Glass transition temperature of soft segments was significantly higher after increasing of R,S-PHB in the PURs structure (
Figure 3). The biggest changes were observed for PURs with the highest number of branching points. As glass temperature of oligomer R,S-PHB was −5.5 °C, its blending with PCL chains increased
Tg of soft segments. Presence of the one
Tg on DSC thermograms indicated on miscibility of these two oligomerols and increasing the R,S-PHB quantity increased the glass transition temperature.
Since the DSC thermograms from the first heating cycle show all changes occurring in polymer structure during sample formation and subsequent storage, it was assumed that lower Tg PUR x/15 compared to PUR x/5 might result from differences in conditions (e.g., conditioning temperature). However, the results from the second heating cycle clearly showed that the addition of more PCLt caused cross-linking of the chains and thus their stiffening, which increased the TgII temperature.
The DSC investigation was conducted about 2 months after the synthesis of PURs. During this time, polyurethane chains slowly reorganized and ordered, resulting in the formation of crystalline forms. Therefore, after cooling a PURs sample at the rate of 10 °C·min
−1, it was found out that no crystalline forms were formed in the polymer network. On thermograms of the second heating cycle, only glass transition of the soft segments was observed. Generally, the values of
Tg from the second heating scan were lower than the ones obtained from the first heating. Only in the case of PUR 0/15 and PUR 20/15, whose melting enthalpy of soft segments was less than 1 J/g,
Tg from the second heating was higher. It was concluded that the presence of crystalline forms hindered mobility of chains, which consequently moved
Tg to higher temperatures (
Table 3).
The analysis of the results of the second heating (after eliminating the thermal history of a sample) confirmed compliance with the results presented in [
11], which indicated that an increase in
Mc resulted in an increment of
Tg. PURs with the small amount of PCL
t and M
c about 24,000 g·mol
−1 had lower
TgII than PURs with higher branching points. Nevertheless, it was concluded that side chains CH
3 and C
2H
5 were too short to have a plasticizing effect, as it was stated in [
11] in the case of side chains in triolein used for PUR synthesise.
Thermal decomposition of PUR was measured by TG and DTG (
Figure 4). In the case of PURs without R,S-PHB a one-step process of thermal degradation was observed. Temperature of the maximum degradation of PUR 0/5 and PUR 0/15 was 356 °C, and 362 °C, respectively (
Table S3, Supplementary Materials). The initial degradation temperature was higher for PUR 0/15 with the increased number of cross-links, which is in agreement with the literature data [
14,
15]. Increasing the amount of PCL
t in the structure of soft polyurethane segments, without R,S-PHB clearly increasing the thermal stability of the sample, indicated an increase in cross-linking. However, the addition of R,S-PHB disrupted cross-linking processes. The lower
Ti and
T5 values of polyurethanes with a higher amount of PCL
t compared to PUR x/5 indicated that the polyurethane network had chains with low molecular weights that underwent thermal degradation faster. In addition, the crosslinked networks of these polyurethanes may contain the residual particles of solvent, which further affect the weight reduction during the TG analysis. Derivative TG curves clearly indicated that PURs with R,S-PHB degraded in three stages. The presence of secondary OH groups in the R,S-PHB structure caused this polyol to be less reactive than PCL
d and PCL
t in reaction with the isocyanate group [
16]. The reactivity of the secondary group is assumed to be only 30% of that of primary alcohols [
17]. In consequence distribution of molecular weight was therefore probably high. The resulting shorter chains probably degraded at a lower temperature than chain with the high molecular weight and the initial temperature (
Ti) of thermal degradation of PURs with R,S-PHB was lower (
Table 3). Such a decrease of degradation temperature with the molecular weight decreasing was observed for poly(dimethylsiloxane) [
18] and poly(methyl methacrylate) [
19]. Knowing that degradation of PUR starts from a breakage of urethane bonds in hard segments [
20], there was a higher intensity of the first peak of the synthetized polyurethanes with R,S-PHB, as the presence of R,S-PHB caused formation of more urethane groups, and temperatures of the initial decomposition and reduction of 5% and 10% samples mass were found to be the lowest. Polyurethanes based on high amount of R,S-PHB (PUR 30/5, PUR 30/20 and PUR 45/20) underwent the fastest thermal degradation (
Ti was the lowest), despite the high number of cross-linking nodes (in case of PUR 30/20 and PUR 45/20). The number of side (CH
3 and C
2H
5) chains in PUR 45/20 structure was the highest (
Table 1). Their presence caused the macrochains to move away from one another, and as a consequence, the interaction between them was reduced, so the thermal stability was also low.
On the surface of the samples with the highest enthalpy of melting of soft segments (
Table 3), the lamellas (marked with white arrows in
Figure 5) that could organize into spherules were visible. The spherules and a clear boundary between them on AFM images of PUR 10/5 and PUR 20/5 were marked with red arrows in
Figure 5. The width of these lamellas was within 10–40 nm. Cross-sectional images indicated that lamellas occurred also throughout the entire sample volume (
Figure S2, Supplementary Materials).
The PUR 0/5 surface was irregular, with inclusions of 700 nm × 500 nm (large inclusions), 140 nm × 220 nm (medium inclusions) and 60 nm × 70 nm–90 nm × 130 nm (fine inclusions) (
Figure 5). On the surface of PURs with the larger amount of PCL
t in soft segments, a few inclusions and the small number of lamellase (marked with white arrows) of the width of 10–20 nm were observed (e.g., PUR 0/15 and PUR 30/20). However, they were not arranged into any crystalline forms, as it was confirmed by the DSC investigation (low melting enthalpy was observed for these polymers) (
Table 3).
As it is well-known, darker zones in the AFM phase images correspond to the amorphous phase of PURs, while brighter zones correspond to the crystalline phase of these PURs. Taking this into consideration, the AFM results can be correlated with the DSC results [
21], the AFM phase images of polyurethanes indicate that PUR x/15 samples were the least crystalline material.
Introducing R,S-PHB into the structure of PURs with low branching points (PUR x/5) significantly reduced root mean square roughness (R
q) from 85 nm for PUR 0/5 to even 14 nm in the case of PUR 30/5 (
Table S4, Supplementary Materials). The effect of R,S-PHB on roughness was much smaller in the case of PURs with more branching nodes.
Images of the surface in the microscopic scale (under the optical microscope) were in good agreement with AFM observations. On the surface of PUR x/5 with tendency to crystallisation, the characteristic circular objects were visible. Surface topography of other polyurethanes was smooth with no pores or protrusions, but with fine, heterogeneous inclusions.
The contact angle of the synthetized polyurethanes was high, near hydrophobic materials. However, as depicted in
Figure 6 the contact angle observed after 3 min from immersing of a water drop on the polymer surface was significantly reduced. This phenomenon was observed also for polyurethanes without R,S-PHB, but changes in these case were lower. This indicated affinity of these polymers to water.
Samples with higher roughness should supposedly have a lower contact angle [
22]. However, the obtained results did not confirm this. Also, the expected increase in water contact, due to the increased cross-links, was not found [
23]. An increase in the amount of PCL
t in the structure of soft segments (at the same time reduction in M
c) from 5 wt % to 15 wt % increased the contact angle, whereas a further increase in the amount of triol reduced it (
Figure 6 and
Table S4). The highest contact angle was observed for the second series of PUR with 15 wt % PCL
t. It was clearly seen that polyurethanes without R,S-PHB were characterized by the highest contact angle.
It is believed that after film formation, on the sample contact surface with air, were mainly PCL chains because of their hydrophobicity. However, as it was said before, after 3 min from the placement of the water drop, the angle decreased, which indicated an increase in the affinity of polyurethanes to water. These changes were higher for PURs with R,S-PHB. This was due to the higher hydrophilicity of the R,S-PHB chains than PCL, as there was more carbonyl groups capable to forming hydrogen bonds with water in R,S-PHB. Thus, hydrophilic R,S-PHB chains migrated to the sample surface under the influence of water droplets.
In case of almost linear PUR x/5, the ordering of chains and crystallinity was the highest, and the free migration of PCL chains to the surface was limited. The presence of R,S-PHB on PURs surface was observed by ATR-FTIR analysis. In contrast, the interaction in PUR x/15 between the chains was much smaller (which was confirmed by the DSC results) due to greater number of branches so the hydrophobic PCL chains could easily move to the surface of the sample, and the contact angle increased.
However, with the highest quantity of PCLt, polyurethanes were cross-linked, which resulted in reduced polycaprolactone chains mobility during the sample formation, hence the reduction of the contact angle to values similar to PUR x/5.
The water affinity of the obtained polyurethanes, observed during the contact angle measurements, was confirmed by a high water uptake. Water absorption percentage visibly increased with an increase in immersion time for all samples, and despite of 21 days of incubation, some samples did not obtain the equilibrium swelling.
The samples of polyurethanes without R,S-PHB increased their weight only by 1.6% after the first day of incubation in water, and this value did not change during investigations (
Figure 7). Undoubtedly, addition of R,S-PHB into the structure of soft segments caused a significant increase in water sorption. The same tendency was observed by Wang and others [
24]. Moreover, the greater the amount of R,S-PHB in the polyurethane network, the greater water sorption. The highest amount of water was absorbed for PUR 45/20 polymer with the soft segments built in almost half with R,S-PHB.
Samples with 5 wt % of PCL
t in soft segments absorbed the lowest amount of water. The highest
Mc of chains between branch points was connected with the highest possibility of interaction (such as hydrogen bonds) between long aliphatic polyurethane chains and water molecules hardly penetrated inside the bulk sample. As the content of side CH
3 groups increased (
Table 1), those interactions were inhibited, which facilitated penetration of water into the polyurethane network. Meanwhile, when the amount of PCL
t increased, the polymer with large free spaces, limited through cross-linking, was obtained. Relatively long chains between the network nodes (high
Mc) caused that water could penetrate. Hence, an increase in water sorption was observed in the case of PUR x/15. However, when the number of cross-links was too high (as in the case of PUR x/20), water molecules could not penetrate deep into the network.
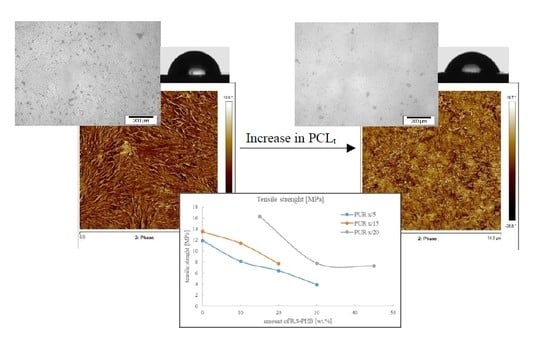
The relation between tensile strength and the amount of R,S-PHB in the structure of soft segments and values in samples hardness are depicted in
Figure 8.
Tensile strength values were not high, but they were still higher than for example in the case of polyurethane composite with natural PHB used as reinforcement [
25]. Two significant parameters affected tensile strength: The number of cross-links (introduced with PCL
t) and the amount of R,S-PHB in soft segments. In all PUR series there was a trend of a decrease in tensile strength with an increase of R,S-PHB in the network (
Figure 8). It was supposed that the reason was the presence of a secondary group in the R,S-PHB chain, which hindered the reaction with NCO groups; and consequently, the structure was not uniform and statistically repeatable. This caused worse ordering of individual segments, and thus, reduced the interaction between chains and deteriorated mechanical properties. Wang and others found that by introducing a higher amount of natural polyhydroxybutarate diol (also with a secondary hydroxyl group), the tensile strength of polyurethanes proved to be higher [
24]. Thus, the amorphous R,S-PHB affect the PURs properties in different way than crystalline R-PHB. We suppose that the crystalline nature of natural PHB could be the reason this increased strength.
The number of branching nodes was another considered factor that affected mechanical strength. Increasing the amount of cross-linking by using more quantity of PCL
t and reducing M
c, between cross-linking nodes, increased tensile strength of polyurethanes, which was expected [
26].
The hardness of PURs samples was high and slightly decreased after R,S-PHB adding. At the same time hardness of samples increased after increasing the amount of triol (
Figure 8 and
Table S5, Supplementary Materials). The increasing of polyurethane hardness with increasing in cross-links number is well known [
26]. However, it was expected that differences between hardness of branched PURs and samples with higher amount of PCL
t would be more significant. Moreover, hardness of the samples with low branching nodes should be lower. On the other hand, the highest PUR x/5 crystallinity was antagonistic to the effect of the highest linearity of these polyurethanes and, consequently, hardness did not degrease as much, as it was expected.