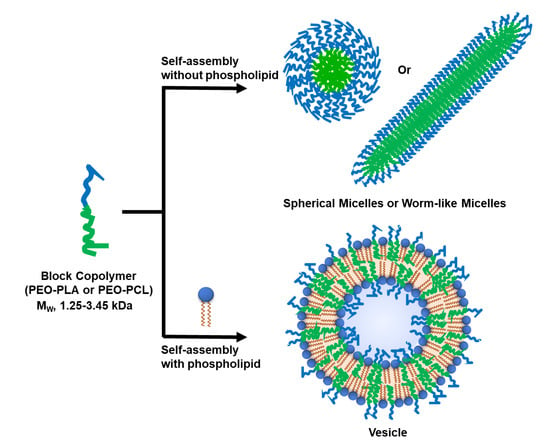
Facile Mixing of Phospholipids Promotes Self-Assembly of Low-Molecular-Weight Biodegradable Block Co-Polymers into Functional Vesicular Architectures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Large Unilamellar Vesicles (LUVs)
2.3. Dynamic Light Scattering (DLS)
2.4. Cryogenic-Transmission Electron Microscopy (Cryo-TEM)
2.5. Calcein Leakage Assay
2.6. Preparation of Giant Unilamellar Vesicles (GUVs)
2.7. Wide-Field Deconvolution Microscopy
2.8. Phospholipase A2 Enzymatic Assay
3. Results and Discussion
3.1. Selection of Block Co-Polymers (BCPs)
3.2. Formation of Hybrid POPC/BCP Large Unilamellar Vesicles
3.3. Surface Topography of POPC/BCP Giant Unilamellar Vesicles
3.4. Surface Topography of Hybrid POPC/BCP/Cholesterol Giant Unilamellar Vesicles
3.5. Enzymatic Activity on Hybrid POPC/BCP Large Unilamellar Vesicles
3.6. Enzymatic Activity on Hybrid POPC/BCP Giant Unilamellar Vesicles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Bio. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Nicolson, G.L. The Fluid-Mosaic Model of Membrane Structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1451–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifert, U. Configurations of fluid membranes and vesicles. Adv. Phys. 1997, 46, 13–137. [Google Scholar] [CrossRef]
- Rawicz, W.; Olbrich, K.C.; McIntosh, T.; Needham, D.; Evans, E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 2000, 79, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Holthuis, J.C.M.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Torok, Z.; Crul, T.; Maresca, B.; Schutz, G.J.; Viana, F.; Dindia, L.; Piotto, S.; Brameshuber, M.; Balogh, G.; Peeter, M.; et al. Plasma membranes as heat stress sensors: From lipid-controlled molecular switches to therapeutic applications. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1594–1618. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Chirico, S. Lipid-Peroxidation—Its Mechanism, Measurement, and Significance. Am. J. Clin. Nutr. 1993, 57, 715–725. [Google Scholar] [CrossRef] [Green Version]
- de Haas, K.H.; Blom, C.; van den Ende, D.; Duits, M.H.G.; Mellema, J. Deformation of giant lipid bilayer vesicles in shear flow. Phys. Rev. E 1997, 56, 7132–7137. [Google Scholar] [CrossRef] [Green Version]
- Needham, D.; Nunn, R.S. Elastic-Deformation and Failure of Lipid Bilayer-Membranes Containing Cholesterol. Biophys. J. 1990, 58, 997–1009. [Google Scholar] [CrossRef] [Green Version]
- Bates, F.S.; Fredrickson, G.H. Block copolymers—Designer soft materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Discher, B.M.; Won, Y.Y.; Ege, D.S.; Lee, J.C.M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [Green Version]
- Le Meins, J.F.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Schulz, M.; Binder, W.H. Mixed Hybrid Lipid/Polymer Vesicles as a Novel Membrane Platform. Macromol. Rapid Commun. 2015, 36, 2031–2041. [Google Scholar] [CrossRef]
- Lian, T.; Ho, R.J.Y. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Santore, M.M. Hybrid copolymer-phospholipid vesicles: Phase separation resembling mixed phospholipid lamellae, but with mechanical stability and control. Soft Matter 2015, 11, 2617–2626. [Google Scholar] [CrossRef]
- Khan, S.; Li, M.; Muench, S.P.; Jeuken, L.J.; Beales, P.A. Durable proteo-hybrid vesicles for the extended functional lifetime of membrane proteins in bionanotechnology. Chem. Commun. 2016, 52, 11020–11023. [Google Scholar] [CrossRef] [Green Version]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.Y.; Car, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef] [Green Version]
- Dao, T.P.; Fernandes, F.; Ibarboure, E.; Ferji, K.; Prieto, M.; Sandre, O.; Le Meins, J.F. Modulation of phase separation at the micron scale and nanoscale in giant polymer/lipid hybrid unilamellar vesicles (GHUVs). Soft Matter 2016. [Google Scholar] [CrossRef] [Green Version]
- Dao, T.P.T.; Fernandes, F.; Er-Rafik, M.; Salva, R.; Schmutz, M.; Brulet, A.; Prieto, M.; Sandre, O.; Le Meins, J.F. Phase Separation and Nanodomain Formation in Hybrid Polymer/Lipid Vesicles. ACS Macro Lett. 2015, 4, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Heberle, F.A.; Petruzielo, R.S.; Pan, J.J.; Drazba, P.; Kucerka, N.; Standaert, R.F.; Feigenson, G.W.; Katsaras, J. Bilayer Thickness Mismatch Controls Domain Size in Model Membranes. Biophys. J. 2014, 106, 288a. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.; Vanderlick, T.K.; Beales, P.A. Formation and dissolution of phospholipid domains with varying textures in hybrid lipo-polymersomes. Soft Matter 2012, 8, 7982–7988. [Google Scholar] [CrossRef]
- Nam, J.; Beales, P.A.; Vanderlick, T.K. Giant phospholipid/block copolymer hybrid vesicles: Mixing behavior and domain formation. Langmuir 2011, 27, 1–6. [Google Scholar] [CrossRef]
- LoPresti, C.; Massignani, M.; Fernyhough, C.; Blanazs, A.; Ryan, A.J.; Madsen, J.; Warren, N.J.; Armes, S.P.; Lewis, A.L.; Chirasatitsin, S.; et al. Controlling Polymersome Surface Topology at the Nanoscale by Membrane Confined Polymer/Polymer Phase Separation. ACS Nano 2011, 5, 1775–1784. [Google Scholar] [CrossRef]
- Olubummo, A.; Schulz, M.; Schops, R.; Kressler, J.; Binder, W.H. Phase Changes in Mixed Lipid/Polymer Membranes by Multivalent Nanoparticle Recognition. Langmuir 2014, 30, 259–267. [Google Scholar] [CrossRef]
- Ruysschaert, T.; Sonnen, A.F.P.; Haefele, T.; Meier, W.; Winterhaltert, M.; Fournier, D. Hybrid nanocapsules: Interactions of ABA block copolymers with liposomes. J. Am. Chem. Soc. 2005, 127, 6242–6247. [Google Scholar] [CrossRef]
- Kang, J.Y.; Choi, I.; Seo, M.; Lee, J.Y.; Hong, S.; Gong, G.; Shin, S.S.; Lee, Y.; Kim, J.W. Enhancing membrane modulus of giant unilamellar lipid vesicles by lateral co-assembly of amphiphilic triblock copolymers. J. Colloid Interface Sci. 2020, 561, 318–326. [Google Scholar] [CrossRef]
- Pippa, N.; Kaditi, E.; Pispas, S.; Demetzos, C. PEO-b-PCL-DPPC chimeric nanocarriers: Self-assembly aspects in aqueous and biological media and drug incorporation. Soft Matter 2013, 9, 4073–4082. [Google Scholar] [CrossRef]
- Pippa, N.; Pispas, S.; Demetzos, C. The metastable phases as modulators of biophysical behavior of liposomal membranes. J. Therm. Anal. Calorim. 2015, 120, 937–945. [Google Scholar] [CrossRef]
- Palominos, M.A.; Vilches, D.; Bossel, E.; Soto-Arriaza, M.A. Interaction between amphipathic triblock copolymers and L-α-dipalmitoyl phosphatidylcholine large unilamellar vesicles. Colloids Surf. B Biointerfaces 2016, 148, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Naziris, N.; Stellas, D.; Massala, C.; Zouliati, K.; Pispas, S.; Demetzos, C.; Forys, A.; Marcinkowski, A.; Trzebicka, B. PEO-b-PCL grafted niosomes: The cooperativilty of amphiphilic components and their properties in vitro and in vivo. Colloids Surf. B Biointerfaces 2019, 177, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; McCabe, J.; Hill, K.; Beales, P.A. Biodegradable hybrid block copolymer—Lipid vesicles as potential drug delivery systems. J. Colloid Interface Sci. 2020, 562, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Wong, A.; De Hoog, H.-P.M.; Rangamani, P.; Parikh, A.; Nallani, M.; Sandin, S.; Liedberg, B. Spontaneous formation of nanometer scale tubular vesicles in aqueous mixtures of lipid and block copolymer amphiphiles. Soft Matter 2016. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.G.; Zhu, W.W.; Venegas, B. On the lateral structure of model membranes containing cholesterol. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Angelova, M.I.; Dimitrov, D.S. Liposome Electroformation. Faraday Discuss. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Aranda-Espinoza, H.; Bermudez, H.; Bates, F.S.; Discher, D.E. Electromechanical limits of polymersomes. Phys. Rev. Lett. 2001, 87. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of PEG-PLA and PEG-PCL: Hydrolysis-triggered controlled release vesicles. J. Control. Release 2004, 96, 37–53. [Google Scholar] [CrossRef]
- Thoma, J.; Belegrinou, S.; Rossbach, P.; Grzelakowski, M.; Kita-Tokarczyk, K.; Meier, W. Membrane protein distribution in composite polymer-lipid thin films. Chem. Commun. 2012, 48, 8811–8813. [Google Scholar] [CrossRef]
- Kowal, J.; Wu, D.L.; Mikhalevich, V.; Palivan, C.G.; Meier, W. Hybrid Polymer-Lipid Films as Platforms for Directed Membrane Protein Insertion. Langmuir 2015, 31, 4868–4877. [Google Scholar] [CrossRef]
- Baumgart, T.; Hammond, A.T.; Sengupta, P.; Hess, S.T.; Holowka, D.A.; Baird, B.A.; Webb, W.W. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 2007, 104, 3165–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgart, T.; Hess, S.T.; Webb, W.W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 2003, 425, 821–824. [Google Scholar] [CrossRef]
- Holme, M.N.; Fedotenko, I.A.; Abegg, D.; Althaus, J.; Babel, L.; Favarger, F.; Reiter, R.; Tanasescu, R.; Zaffalon, P.L.; Ziegler, A.; et al. Shear-stress sensitive lenticular vesicles for targeted drug delivery. Nat. Nanotechnol. 2012, 7, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H. Polyhedral vesicles: A Brownian dynamics simulation. Phys. Rev. E 2003, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Zou, T.; Tao, X.; Semetey, V.; Trepout, S.; Marco, S.; Ling, J.; Li, M.H. Poly(epsilon-caprolactone)-block-polysarcosine by Ring-Opening Polymerization of Sarcosine N-Thiocarboxyanhydride: Synthesis and Thermoresponsive Self-Assembly. Biomacromolecules 2015, 16, 3265–3274. [Google Scholar] [CrossRef]
- Gao, K.J.; Liu, X.Z.; Li, G.T.; Xu, B.Q.; Yi, J.J. Spontaneous formation of giant vesicles with tunable sizes based on jellyfish-like graft copolymers. RSC Adv. 2014, 4, 59323–59330. [Google Scholar] [CrossRef]
- Baumgart, T.; Das, S.; Webb, W.W.; Jenkins, J.T. Membrane Elasticity in Giant Vesicles with Fluid Phase Coexistence. Biophys. J. 2005, 89, 1067–1080. [Google Scholar] [CrossRef] [Green Version]
- Arouri, A.; Mouritsen, O.G. Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipid Res. 2013, 52, 130–140. [Google Scholar] [CrossRef]
- Georgieva, R.; Mircheva, K.; Vitkova, V.; Balashev, K.; Ivanova, T.; Tessier, C.; Koumanov, K.; Nuss, P.; Momchilova, A.; Staneva, G. Phospholipase A2-Induced Remodeling Processes on Liquid-Ordered/Liquid-Disordered Membranes Containing Docosahexaenoic or Oleic Acid: A Comparison Study. Langmuir 2016, 32, 1756–1770. [Google Scholar] [CrossRef]
- Tanaka, T.; Sano, R.; Yamashita, Y.; Yamazaki, M. Shape changes and vesicle fission of giant unilamellar vesicles of liquid-ordered phase membrane induced by lysophosphatidylcholine. Langmuir 2004, 20, 9526–9534. [Google Scholar] [CrossRef]
- Zhelev, D.V. Material property characteristics for lipid bilayers containing lysolipid. Biophys. J. 1998, 75, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, K.; Davidsen, J.; Mouritsen, O.G. Biophysical mechanisms of phospholipase A2 activation and their use in liposome-based drug delivery. FEBS Lett. 2002, 531, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, F.; Jain, M.K. Phospholipase-A2 at the Bilayer Interface. Proteins 1991, 9, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Winget, J.M.; Pan, Y.H.; Bahnson, B.J. The interfacial binding surface of phospholipase A2s. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2006, 1761, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Callisen, T.H.; Talmon, Y. Direct imaging by cryo-TEM shows membrane break-up by phospholipase A(2) enzymatic activity. Biochemistry 1998, 37, 10987–10993. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Cho, N.J.; Duran, R.S.; Frank, C.W. Interfacial binding dynamics of bee venom phospholipase A2 investigated by dynamic light scattering and quartz crystal microbalance. Langmuir 2010, 26, 4103–4112. [Google Scholar] [CrossRef]
- Moroz, J.D.; Nelson, P.; BarZiv, R.; Moses, E. Spontaneous expulsion of giant lipid vesicles induced by laser tweezers. Phys. Rev. Lett. 1997, 78, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Hermann, P.M.; Watson, S.N.; Wildering, W.C. Phospholipase A(2)—Nexus of aging, oxidative stress, neuronal excitability, and functional decline of the aging nervous system? Insights from a snail model system of neuronal aging and age-associated memory impairment. Front. Genet. 2014, 5. [Google Scholar] [CrossRef]
- Wick, R.; Angelova, M.I.; Walde, P.; Luisi, P.L. Microinjection into giant vesicles and light microscopy investigation of enzyme-mediated vesicle transformations. Chem. Biol. 1996, 3, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Burack, W.R.; Dibble, A.R.G.; Allietta, M.M.; Biltonen, R.L. Changes in vesicle morphology induced by lateral phase separation modulate phospholipase A(2) activity. Biochemistry 1997, 36, 10551–10557. [Google Scholar] [CrossRef]
- Zimmerberg, J.; Kozlov, M.M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 2006, 7, 9–19. [Google Scholar] [CrossRef]
- Mumtaz Virk, M.; Reimhult, E. Phospholipase A2-Induced Degradation and Release from Lipid-Containing Polymersomes. Langmuir 2018, 34, 395–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.M.; Li, C.S.; Sheng, Y.J.; Wu, D.T.; Tsao, H.K. Size-Dependent Properties of Small Unilamellar Vesicles Formed by Model Lipids. Langmuir 2012, 28, 689–700. [Google Scholar] [CrossRef]
- Lipowsky, R. Coupling of bending and stretching deformations in vesicle membranes. Adv. Colloid Interface Sci. 2014, 208, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.H.; Epstein, T.M.; Jain, M.K.; Bahnson, B.J. Five coplanar anion binding sites on one face of phospholipase A(2). Relationship to interface binding. Biochemistry 2001, 40, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Assender, H.; Bliznyuk, V.; Porfyrakis, K. How surface topography relates to materials properties. Science 2002, 297, 973–976. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Pastor, R.W. A coarse-grained model for PEGylated lipids: The effect of PEGylation on size and shape of self-assembled structures. J. Phys. Chem. B 2011, 115, 7830–7837. [Google Scholar] [CrossRef] [Green Version]





| Formulation. | Composition | POPC:BCP (mol:mol) | Mol. Wt (g/mol) | fhydrophilic | PDI | ||
|---|---|---|---|---|---|---|---|
| Mn | MA | MB | |||||
| Lipid | |||||||
| POPC | POPC | 1:0 | 760 | ||||
| BCP | |||||||
| PCL0.7 | PEG12-PCL6 | 0:1 | 1250 | 550 | 700 | 0.44 | 1.13 |
| PCL1.1 | PEG12-PCL9 | 0:1 | 1650 | 550 | 1100 | 0.33 | 1.17 |
| PLA2.75 | PEG16-PLA38 | 0:1 | 3450 | 700 | 2750 | 0.20 | 1.12 |
| PBD1.2 | PEG13-PBD22 | 0:1 | 1800 | 600 | 1200 | 0.33 | 1.09 |
| Hybrid (POPC/BCP or POPC/BCP/Ch) | |||||||
| POPC/PCL0.7 | POPC: PEG12-PCL6 | 1:1 | |||||
| POPC/PCL1.1 | POPC: PEG12-PCL9 | 1:1 | |||||
| POPC/PLA2.75 | POPC: PEG16-PLA38 | 1:1 | |||||
| POPC/PBD1.2 | POPC: PEG13-PBD22 | 1:1 | |||||
| POPC/PCL0.7/Ch | POPC: PEG12-PCL6: Cholesterol | 1:1:1 | |||||
| POPC/PCL1.1/Ch | POPC: PEG12-PCL9: Cholesterol | 1:1:1 | |||||
| POPC/PLA2.75/Ch | POPC: PEG16-PLA38: Cholesterol | 1:1:1 | |||||
| POPC/PBD1.2/Ch | POPC: PEG13-PBD22: Cholesterol | 1:1:1 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.K.; Ho, J.C.S.; Roy, S.; Liedberg, B.; Nallani, M. Facile Mixing of Phospholipids Promotes Self-Assembly of Low-Molecular-Weight Biodegradable Block Co-Polymers into Functional Vesicular Architectures. Polymers 2020, 12, 979. https://doi.org/10.3390/polym12040979
Khan AK, Ho JCS, Roy S, Liedberg B, Nallani M. Facile Mixing of Phospholipids Promotes Self-Assembly of Low-Molecular-Weight Biodegradable Block Co-Polymers into Functional Vesicular Architectures. Polymers. 2020; 12(4):979. https://doi.org/10.3390/polym12040979
Chicago/Turabian StyleKhan, Amit Kumar, James C. S. Ho, Susmita Roy, Bo Liedberg, and Madhavan Nallani. 2020. "Facile Mixing of Phospholipids Promotes Self-Assembly of Low-Molecular-Weight Biodegradable Block Co-Polymers into Functional Vesicular Architectures" Polymers 12, no. 4: 979. https://doi.org/10.3390/polym12040979