Synthesis and Aggregation Behavior of Temperature- and pH-Responsive Glycopolymers as Sugar-Displaying Conjugates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Synthesis of Glycopolymers
2.4. Transmittance Analysis
2.5. Detection of Saccharide on the Surface of Aggregates
3. Results and Discussion
3.1. Synthesis of Glycopolymers
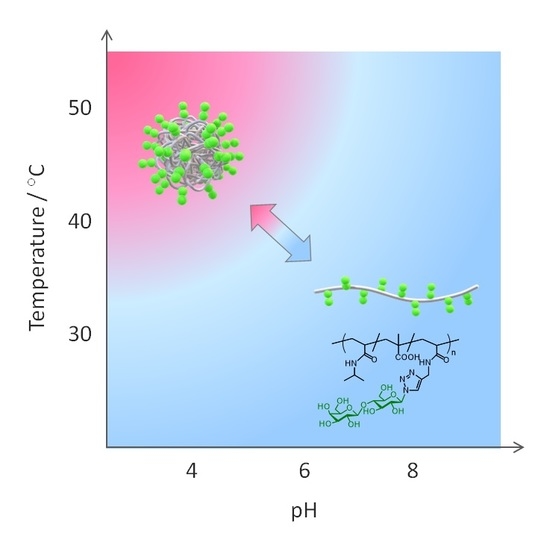
3.2. Aggregation Behavior of Glycopolymers in Aqueous Media
3.3. Detection of Saccharide on the Surface of the Aggregates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Las Heras Alarcón, C.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.K.; Kasi, R.M.; Kim, S.C.; Sharma, N.; Zhou, Y. Stimuli-responsive polymer gels. Soft Matter 2008, 4, 1151–1157. [Google Scholar] [CrossRef]
- Li, M.H.; Keller, P. Stimuli-responsive polymer vesicles. Soft Matter 2009, 5, 927–937. [Google Scholar] [CrossRef]
- Zhai, L. Stimuli-responsive polymer films. Chem. Soc. Rev. 2013, 42, 7148–7160. [Google Scholar] [CrossRef]
- Moad, G. RAFT polymerization to form stimuli-responsive polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Yuk, H.; Cho, S.H.; Lee, S.H. pH/Temperature-Responsive Polymer Composed of Poly((N,N-dimethylamino)ethyl methacrylate-co-ethylacrylamide). Macromolecules 1997, 30, 6856–6859. [Google Scholar] [CrossRef]
- Tian, P.; Wu, Q.; Lian, K. Preparation of temperature- and pH-sensitive, stimuli-responsive poly(N-isopropylacrylamide-co-methacrylic acid) nanoparticles. J. Appl. Polym. Sci. 2008, 108, 2226–2232. [Google Scholar] [CrossRef]
- Chikh Alard, I.; Soubhye, J.; Berger, G.; Gelbcke, M.; Spassov, S.; Amighi, K.; Goole, J.; Meyer, F. Triple-stimuli responsive polymers with fine tuneable magnetic responses. Polym. Chem. 2017, 8, 2450–2456. [Google Scholar] [CrossRef]
- Qiao, X.G.; Dugas, P.Y.; Charleux, B.; Lansalot, M.; Bourgeat-Lami, E. Nitroxide-mediated polymerization-induced self-assembly of amphiphilic block copolymers with a pH/temperature dual sensitive stabilizer block. Polym. Chem. 2017, 8, 4014–4029. [Google Scholar] [CrossRef]
- Brooks, W.L.A.; Vancoillie, G.; Kabb, C.P.; Hoogenboom, R.; Sumerlin, B.S. Triple responsive block copolymers combining pH-responsive, thermoresponsive, and glucose-responsive behaviors. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2309–2317. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Synthesis and Characterization of Thermo-and Chemomechanically Responsive PolyCZV-isopropylacrylamide-co-methacrylic acid) Hydrogels. Macromolecules 1995, 28, 8016–8020. [Google Scholar] [CrossRef]
- Zhang, J.; Peppas, N.A. Molecular interactions in poly(methacrylic acid)/poly(N-isopropyl acrylamide) interpenetrating polymer networks. J. Appl. Polym. Sci. 2001, 82, 1077–1082. [Google Scholar] [CrossRef]
- Lafourcade, C.; Sobo, K.; Kieffer-Jaquinod, S.; Garin, J.; van der Goot, F.G. Regulation of the V-ATPase along the Endocytic Pathway Occurs through Reversible Subunit Association and Membrane Localization. PLoS ONE 2008, 3, e2758. [Google Scholar] [CrossRef] [Green Version]
- Ro, B.I.; Dawson, T.L. The Role of Sebaceous Gland Activity and Scalp Microfloral Metabolism in the Etiology of Seborrheic Dermatitis and Dandruff. J. Investig. Dermatol. Symp. Proc. 2005, 10, 194–197. [Google Scholar] [CrossRef] [Green Version]
- Slavin, S.; Burns, J.; Haddleton, D.M.; Becer, C.R. Synthesis of glycopolymers via click reactions. Eur. Polym. J. 2011, 47, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Sunasee, R.; Narain, R. Glycopolymers and Glyco-nanoparticles in Biomolecular Recognition Processes and Vaccine Development. Macromol. Biosci. 2013, 13, 9–27. [Google Scholar] [CrossRef]
- Ahmed, M.; Wattanaarsakit, P.; Narain, R. Recent advances in the preparation of glycopolymer bioconjugates. Eur. Polym. J. 2013, 49, 3010–3033. [Google Scholar] [CrossRef]
- Miura, Y.; Hoshino, Y.; Seto, H. Glycopolymer nanobiotechnology. Chem. Rev. 2016, 116, 1673–1692. [Google Scholar] [CrossRef]
- Dan, K.; Ghosh, S. PH-responsive aggregation of amphiphilic glyco-homopolymer. Macromol. Rapid Commun. 2012, 33, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Han, Y.; Cheng, C.; Li, C. pH- and glucose-sensitive glycopolymer nanoparticles based on phenylboronic acid for triggered release of insulin. Carbohydr. Polym. 2012, 89, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, T.; Tang, X.; Zhang, Q.; Yu, F.; Pei, M. Triple stimuli-responsive amphiphilic glycopolymer. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2131–2138. [Google Scholar] [CrossRef]
- Yilmaz, G.; Guler, E.; Geyik, C.; Demir, B.; Ozkan, M.; Odaci Demirkol, D.; Ozcelik, S.; Timur, S.; Remzi Becer, C. PH responsive glycopolymer nanoparticles for targeted delivery of anti-cancer drugs. Mol. Syst. Des. Eng. 2018, 3, 150–158. [Google Scholar] [CrossRef]
- Tanaka, T. Protecting-Group-Free Synthesis of Glycomonomers and Glycopolymers from Free Saccharides. Trends Glycosci. Glycotechnol. 2016, 28, E101–E108. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Ishitani, H.; Miura, Y.; Oishi, K.; Takahashi, T.; Suzuki, T.; Shoda, S.I.; Kimura, Y. Protecting-group-free synthesis of glycopolymers bearing sialyloligosaccharide and their high binding with the influenza virus. ACS Macro Lett. 2014, 3, 1074–1078. [Google Scholar] [CrossRef]
- Tanaka, T.; Zhou, Y.; Tamoto, C.; Kurebayashi, Y.; Takahashi, T.; Suzuki, T. An α2,3-Linked Sialylglycopolymer as a Multivalent Glycoligand against Avian and Human Influenza Viruses. J. Appl. Glycosci. 2017, 64, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Nagai, H.; Noguchi, M.; Kobayashi, A.; Shoda, S. One-step conversion of unprotected sugars to β-glycosyl azides using 2-chloroimidazolinium salt in aqueous solution. Chem. Commun. 2009, 3378–3379. [Google Scholar] [CrossRef]
- Shoda, S. Development of chemical and chemo-enzymatic glycosylations. Proc. Japan Acad. Ser. B Phys. Biol. Sci. 2017, 93, 125–145. [Google Scholar] [CrossRef] [Green Version]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chemie Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Hales, M.; Barner-Kowollik, C.; Davis, T.P.; Stenzel, M.H. Shell-cross-linked vesicles synthesized from block copolymers of poly(d,l-lactide) and poly(N-isopropyl acrylamide) as thermoresponsive nanocontainers. Langmuir 2004, 20, 10809–10817. [Google Scholar] [CrossRef] [PubMed]
- von der Ehe, C.; Czaplewska, J.A.; Gottschaldt, M.; Schubert, U.S. Synthesis of thermoresponsive glycopolymers via ATRP of N-isopropylacrylamide and N-allylacrylamide and subsequent thiol–ene reaction. Eur. Polym. J. 2013, 49, 2660–2669. [Google Scholar] [CrossRef]
- Tanaka, T.; Okamoto, M. Reversible temperature-responsive and lectin-recognizing glycosylated block copolymers synthesized by RAFT polymerization. Polym. J. 2018, 50, 523–531. [Google Scholar] [CrossRef]







| Polymer | Feeding Molar Ratio of NIPAM/MAA/LacAAm | Conv. (%) a | Yield (%) b | Mn (g mol−1) a | Mw/Mnc | Unit Ratio of NIPAM/MAA/LacAAm in Polymer a |
|---|---|---|---|---|---|---|
| P1 | 95.0/5.0/0 | 75 | 76 | 14,300 | 1.27 | 94.9/5.1/0 |
| P2 | 93.1/4.9/2.0 | 86 | 76 | 13,400 | 1.30 | 93.6/4.6/1.8 |
| P3 | 90.2/4.8/5.0 | 90 | 72 | 16,600 | 1.33 | 90.5/4.5/5.0 |
| P4 | 85.5/4.5/10.0 | 85 | 50 | 17,300 | 1.32 | 87.1/4.0/8.9 |
| Polymer | pH 4 | pH 5 | pH 6 | pH 7 | pH 8 |
|---|---|---|---|---|---|
| P1 | 26.6 | 27.6 | 32.8 | ND a | ND |
| P2 | 28.6 | 30.1 | 37.1 | ND | ND |
| P3 | 35.4 | 38.1 | 45.7 | ND | ND |
| P4 | 43.9 | 47.6 | ND | ND | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, S.; Aoki, T.; Ushio, S.; Tanaka, T. Synthesis and Aggregation Behavior of Temperature- and pH-Responsive Glycopolymers as Sugar-Displaying Conjugates. Polymers 2020, 12, 956. https://doi.org/10.3390/polym12040956
Tsuji S, Aoki T, Ushio S, Tanaka T. Synthesis and Aggregation Behavior of Temperature- and pH-Responsive Glycopolymers as Sugar-Displaying Conjugates. Polymers. 2020; 12(4):956. https://doi.org/10.3390/polym12040956
Chicago/Turabian StyleTsuji, Sotaro, Tomohiro Aoki, Shunsuke Ushio, and Tomonari Tanaka. 2020. "Synthesis and Aggregation Behavior of Temperature- and pH-Responsive Glycopolymers as Sugar-Displaying Conjugates" Polymers 12, no. 4: 956. https://doi.org/10.3390/polym12040956