Alginate-Edible Coatings for Application on Wild Andean Blueberries (Vaccinium meridionale Swartz): Effect of the Addition of Nanofibrils Isolated from Cocoa By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
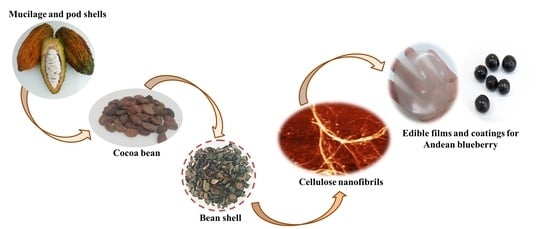
2.2. Isolation of Cellulose Nanofibrils (CNF) from Cocoa Byproducts
2.3. Atomic Force Microscopy
2.4. Preparation of Film/Coating Solutions
2.5. Formation and Characterization of Edible Films
2.6. Application of Edible Coatings
2.7. Evaluation of Quality Attributes of Andean Blueberries along Storage
2.7.1. Respiration Rate
2.7.2. Weight Loss
2.7.3. Soluble Solids Content, pH, and Titratable Acidity (%)
2.7.4. Firmness Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Cellulose Nanofibrils (CNFs) from Cocoa Byproducts
3.2. Film Characterization
3.3. Behavior of Andean Blueberry Quality Parameters during Storage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celis, M.E.M.; Franco Tobón, Y.N.; Agudelo, C.; Arango, S.S.; Rojano, B. Andean Berry (Vaccinium meridionale Swartz). In Fruit and Vegetable Phytochemicals: Chemestry and Human Health, 2nd ed.; Yahia, E.M., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; Volume 2, pp. 869–882. [Google Scholar]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Rincón Soledad, M.C.; Buitrago Guacaneme, C.M.; Ligarreto Moreno, G.A.; Torres Aponte, W.S.; Balaguera López, H.E. Behavior of Agraz Fruit (Vaccinium meridionale Swartz) Harvested in Different Maturity Stages and Stored Under Refrigeration. Rev. Fac. Nac. Agron. 2012, 65, 6615–6625. [Google Scholar]
- Huynh, N.K.; Wilson, M.D.; Eyles, A.; Stanley, R.A. Recent advances in postharvest technologies to extend the shelf life of blueberries (Vaccinium sp.), raspberries (Rubusidaeus L.) and blackberries (Rubus sp.). J. Berry Res. 2019, 9, 687–707. [Google Scholar] [CrossRef]
- Wüstenberg, T. General Overview of Food Hydrocolloids. In Cellulose and Cellulose Derivatives in the Food industry Fundamentals and Applications; Wüstenberg, T., Ed.; Wiley-VCH: Weinheim, Germany, 2015; pp. 1–68. [Google Scholar]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, H.; Kumcuoglu, S.; Tavman, S. Production of edible coatings with twin-nozzle electrospraying equipment and the effects on shelf-life stability of fresh-cut apple slices. J. Food Process Eng. 2017, e12627. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Aldana-Usme, A. Edible coatings based on sodium alginate and ascorbic acid for application on fresh-cut pineapple (Ananas comosus (L.) Merr). Agron. Colomb. 2020, 37, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.; Martin-Belloso, O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT Food Sci. Technol. 2013, 50, 240–246. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Miranda, K.W.E.; Rosa, M.F.; Nascimento, D.M.; de Moura, M.R. Edible films from alginate-acerola puree reinforced with cellulose whiskers. LWT Food Sci. Technol. 2012, 46, 294–297. [Google Scholar] [CrossRef]
- Souza, L.O.; Lessa, O.A.; Dias, M.C.; Tonoli, G.H.D.; Rezende, D.V.B.; Martins, M.A.; Neves, I.C.O.; de Resende, J.V.; Carvalho, E.E.N.; de Barros Vilas Boas, E.V.; et al. Study of morphological properties and rheological parameters of cellulose nanofibrils of cocoa shell (Theobroma cacao L.). Carbohydr. Polym. 2019, 214, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Gómez, H.C.; Serpa, A.; Velásquez-Cock, J.; Gañán, P.; Castro, C.; Vélez, L.; Zuluaga, R. Vegetable nanocellulose in food science: A review. Food Hydrocoll. 2016, 57, 178–186. [Google Scholar] [CrossRef]
- Pitkänen, M.; Honkalampi, U.; von Wright, A.; Sneck, A.; Hentze, H.-P.; Sievänen, J.; Hiltunen, J.; Hellén, E.K.O. Nanofibrillar cellulose—In vitro study of cytotoxic and genotoxic properties. In Proceedings of the TAPPI—International Conference on Nanotechnology for the Forest Products Industry, Otaniemi, Espoo, Finland, 27–29 September 2010. [Google Scholar]
- López-Córdoba, A.; Castro, G.R.; Goyanes, S. Cellulose-Containing Scaffolds Fabricated by Electrospinning: Applications in Tissue Engineering and Drug Delivery. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, K., Kessler, M.R., Eds.; Scrivener Publishing LLC: Beverly, CA, USA, 2017; Volume 8, pp. 361–388. [Google Scholar]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef] [Green Version]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Serra Bonvehí, J.; Ventura Coll, F. Protein quality assessment in cocoa husk. Food Res. Int. 1999, 32, 201–208. [Google Scholar] [CrossRef]
- El-Saied, H.M.; Morsi, M.K.; Amer, M.M.A. Composition of cocoa shell fat as related to cocoa butter. Z. Ernährungswiss 1981, 20, 145–151. [Google Scholar] [CrossRef]
- Jimat, D.N.; Putra, F.A.; Sulaiman, S.; Nor, Y.A.; Mohamed Azmin, N.F.; Syed Putra, S.S. Physicochemical characteristics of bionanocomposites, polycaprolactone/starch/cocoa pod husk microfibrillated cellulose. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 55, 199–208. [Google Scholar]
- Lubis, M.; Gana, A.; Maysarah, S.; Ginting, M.H.S.; Harahap, M.B. Production of bioplastic from jackfruit seed starch (Artocarpus heterophyllus) reinforced with microcrystalline cellulose from cocoa pod husk (Theobroma cacao L.) using glycerol as plasticizer. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012100. [Google Scholar] [CrossRef]
- Zuluaga, R.; Putaux, J.L.; Cruz, J.; Vélez, J.; Mondragon, I.; Gañán, P. Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features. Carbohydr. Polym. 2009, 76, 51–59. [Google Scholar] [CrossRef]
- Velásquez-Cock, J.; Gañán, P.; Posada, P.; Castro, C.; Serpa, A.; Gómez, H.C.; Putaux, J.-L.; Zuluaga, R. Influence of combined mechanical treatments on the morphology and structure of cellulose nanofibrils: Thermal and mechanical properties of the resulting films. Ind. Crops Prod. 2016, 85, 1–10. [Google Scholar] [CrossRef]
- Velásquez-Cock, J.; Serpa, A.; Vélez, L.; Gañán, P.; Gómez Hoyos, C.; Castro, C.; Duizer, L.; Goff, H.D.; Zuluaga, R. Influence of cellulose nanofibrils on the structural elements of ice cream. Food Hydrocoll. 2019, 87, 204–213. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Villalobos-Carvajal, R.; Hernández-Muñoz, P.; Albors, A.; Chiralt, A. Barrier and optical properties of edible hydroxypropyl methylcellulose coatings containing surfactants applied to fresh cut carrot slices. Food Hydrocoll. 2009, 23, 526–535. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. On the use of carrageenan matrices for the development of antiviral edible coatings of interest in berries. Food Hydrocoll. 2019, 92, 74–85. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Rodoni, L.M.; Guardianelli, L.M.; Chaves, A.R.; Martínez, G.A. Use of LED light for Brussels sprouts postharvest conservation. Sci. Hortic. (Amsterdam) 2016, 213, 281–286. [Google Scholar] [CrossRef]
- Mercado, J.A.; Matas, A.J.; Posé, S. Fruit and Vegetable Texture: Role of Their Cell Walls. In Reference Module in Food Science; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 1–7. ISBN 978-0-12-814045-1. [Google Scholar]
- Martins, M.P.; Dagostin, J.L.A.; Franco, T.S.; de Muñiz, G.I.B.; Masson, M.L. Application of Cellulose Nanofibrils Isolated from an Agroindustrial Residue of Peach Palm in Cassava Starch Films. Food Biophys. 2020. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Estevez-Areco, S.; Goyanes, S.; López-Córdoba, A. Characterization of Starches Isolated from Colombian Native Potatoes and Their Application as Novel Edible Coatings for Wild Andean Blueberries (Vaccinium meridionale Swartz). Polymers 2019, 11, 1937. [Google Scholar] [CrossRef] [Green Version]
- Chironi, S.; Bacarella, S.; Altamore, L.; Ingrassia, M. Quality Factors Influencing Consumer Demand for Small Fruit by Focus Group and Sensory Test. J. Food Prod. Mark. 2017, 23, 857–872. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Olivas, G.I.; Mattinson, D.S.; Barbosa-Cánovas, G. V Alginate coatings for preservation of minimally processed ‘Gala’ apples. Postharvest Biol. Technol. 2007, 45, 89–96. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Bras, J.; Siqueira, G.; Chappey, C.; Lebrun, L.; Khelifi, B.; Marais, S.; Dufresne, A. Water sorption behavior and gas barrier properties of cellulose whiskers and microfibrils films. Carbohydr. Polym. 2011, 83, 1740–1748. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Wang, J.; Gardner, D.J.; Tajvidi, M. Cellulose nanofibrils versus cellulose nanocrystals: Comparison of performance in flexible multilayer films for packaging applications. Food Packag. Shelf Life 2020, 23, 100464. [Google Scholar] [CrossRef]
- Paniagua, A.C.; East, A.R.; Hindmarsh, J.P.; Heyes, J.A. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Silva de Moraes, K.; Fagundes, C.; Melo, M.C.; Andreani, P.; Rodriguez Monteiro, A. Conservation of Williams pear using edible coating with alginate and carrageenan. Food Sci. Technol. 2012, 32, 679–684. [Google Scholar] [CrossRef] [Green Version]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-Layer Electrostatic Deposition of Edible Coating on Fresh Cut Melon Model: Anticipated and Unexpected Effects of Alginate–Chitosan Combination. Food Bioprocess Technol. 2014, 7, 1424–1432. [Google Scholar] [CrossRef]







| Sample | Thickness (mm) | Transparency (%) | Water vapor permeability (g s−1 m−1 Pa−1 × 10−10) |
|---|---|---|---|
| Alginate | 0.23 ± 0.01 a | 8.1 ± 0.4 a | 2.9 × 10−9 ± 0.4 a |
| Alginate/CNFs 0.1% | 0.24 ± 0.02 a | 5.9 ± 0. 2 b | 1.1 × 10−9 ± 0.1 b |
| Alginate/CNFs 0.3% | 0.22 ± 0.01 a | 4.8 ± 0.3 c | 0.8 × 10−9 ± 0.1 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Jaramillo, C.; Quintero-Pimiento, C.; Gómez-Hoyos, C.; Zuluaga-Gallego, R.; López-Córdoba, A. Alginate-Edible Coatings for Application on Wild Andean Blueberries (Vaccinium meridionale Swartz): Effect of the Addition of Nanofibrils Isolated from Cocoa By-Products. Polymers 2020, 12, 824. https://doi.org/10.3390/polym12040824
Medina-Jaramillo C, Quintero-Pimiento C, Gómez-Hoyos C, Zuluaga-Gallego R, López-Córdoba A. Alginate-Edible Coatings for Application on Wild Andean Blueberries (Vaccinium meridionale Swartz): Effect of the Addition of Nanofibrils Isolated from Cocoa By-Products. Polymers. 2020; 12(4):824. https://doi.org/10.3390/polym12040824
Chicago/Turabian StyleMedina-Jaramillo, Carolina, Carmen Quintero-Pimiento, Catalina Gómez-Hoyos, Robin Zuluaga-Gallego, and Alex López-Córdoba. 2020. "Alginate-Edible Coatings for Application on Wild Andean Blueberries (Vaccinium meridionale Swartz): Effect of the Addition of Nanofibrils Isolated from Cocoa By-Products" Polymers 12, no. 4: 824. https://doi.org/10.3390/polym12040824