Structure and Properties of PSf Hollow Fiber Membranes with Different Molecular Weight Hyperbranched Polyester Using Pentaerythritol as Core
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
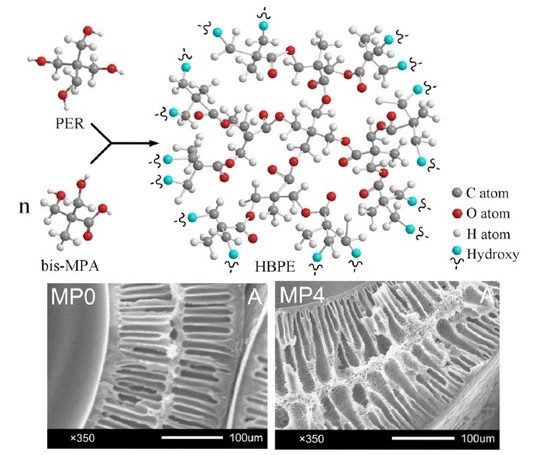
2.2. Synthesis and Characterization of HBPEs
2.3. Preparation of PSf Hollow Fiber Membranes
2.4. Characterization of Casting Solutions
2.4.1. Viscosity Measurements
2.4.2. Light Transmittance Measurement
2.5. Membrane Characterization
2.5.1. Morphology
2.5.2. Hydrophilicity and Porosity
2.5.3. Permeation Property
2.5.4. Pore Size Distribution and Molecular Weight Cut-off (MWCO)
2.5.5. Mechanical Properties
3. Results and Discussion
3.1. Characterization of HBPEs
3.2. Viscosity
3.3. Light Transmittance
3.4. Morphology
3.5. Hydrophilicity and Porosity
3.6. Permeation Performance
3.7. Pore Size, Pore-Size Distribution and MWCO
3.8. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cho, Y.H.; Kim, H.W.; Nam, S.Y.; Park, H.B. Fouling-tolerant polysulfone–poly(ethylene oxide) random copolymer ultrafiltration membranes. J. Membr. Sci. 2011, 379, 296–306. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, Y.; Sun, D.B.; Xiao, C.F.; Zhang, Y.F. Preparation and performance of sulfonated polysulfone flat ultrafiltration membranes. Polym. Eng. Sci. 2015, 55, 1003–1011. [Google Scholar] [CrossRef]
- Li, X.J.; Janke, A.; Formanek, P.; Fery, A.; Stamm, M.; Tripathi, B.P. High permeation and antifouling polysulfone ultrafiltration membranes with in situ synthesized silica nanoparticles. Mater. Today Commun. 2020, 22, 100784. [Google Scholar] [CrossRef]
- Dong, H.B.; Xu, Y.Y.; Yi, Z.; Shi, J.L. Modification of polysulfone membranes via surface-initiated atom transfer radical polymerization. Appl. Surf. Sci. 2009, 255, 8860–8866. [Google Scholar] [CrossRef]
- Yu, H.J.; Cao, Y.M.; Kang, G.D.; Liu, Z.N.; Kuang, W.; Liu, J.H.; Zhou, M.Q. Enhancing the antifouling properties of polysulfone ultrafiltration membranes by the grafting of poly(ethylene glycol) derivatives via surface amidation reactions. J. Appl. Polym. Sci. 2015, 132, 41870. [Google Scholar] [CrossRef]
- Chen, G.E.; Wu, W.Z.; Zhang, P.Y.; Xu, Z.L. Influence of residence time on performance of PVDF membranes prepared via free radical polymerization. J. Appl. Polym. Sci. 2014, 131, 39987. [Google Scholar] [CrossRef]
- Ding, X.L.; Liu, Z.G.; Hua, M.M.; Kang, T.; Li, X.; Zhang, Y.Z. Poly(ethylene glycol) crosslinked sulfonated polysulfone composite membranes for forword osmosis. J. Appl. Polym. Sci. 2016, 133, 43941. [Google Scholar] [CrossRef]
- Hoffmann, C.; Silau, H.; Pinelo, M.; Woodley, J.M.; Daugarrd, A.E. Surface modification of polysulfone membranes applied for a membrane reactor with immobilized alcohol dehydrogenase. Mater. Today Commun. 2018, 14, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Nasef, M.M.; Zubir, N.A.; Ismail, A.F.; Khayet, M.; Dahlan, K.Z.M.; Saidi, H.; Rohani, R.; Ngah, T.I.S.; Sulaiman, N.A. PSSA pore-filled PVDF membranes by simultaneous electron beam irradiation: Preparation and transport characteristics of protons and methanol. J. Membr. Sci. 2006, 268, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Yuenyao, C.; Tirawanichakul, Y.; Chittrakarn, T. Asymmetric polysulfone gas separatin membranes treated by low pressure DC glow discharge plasmas. J. Appl. Polym. Sci. 2015, 132, 42116. [Google Scholar] [CrossRef]
- Ali, F.A.A.; Alam, J.; Shukla, A.K.; Alhoshan, M.; Ansari, M.A.; Al-Masry, W.A.; Rehman, S.; Alam, M. Evaluation of antibacterial and antifouling properties of silver-loaded GO polysulfone nanocomposite membrane against Escherichia coli, Staphylococcus aureus, and BSA protein. React. Funct. Polym. 2019, 140, 136–147. [Google Scholar] [CrossRef]
- Liu, M.; Chen, D.G.; Xu, Z.L.; Wei, Y.M.; Tong, M. Effects of nucleating agents on the morphologies and performances of poly(vinylidene fluoride) microporous membranes via thermally induced phase separation. J. Appl. Polym. Sci. 2013, 128, 836–844. [Google Scholar] [CrossRef]
- Yuan, G.L.; Xu, Z.L.; Wei, Y.M. Characterization of PVDF–PFSA hollow fiber UF blend membrane with low-molecular weight cut-off. Sep. Purif. Technol. 2009, 69, 141–148. [Google Scholar] [CrossRef]
- Guo, J.; Sotto, A.; Martin, A.; Kim, J. Preparation and characterization of polyethersulfone mixed matrix membranes embedded with Ti- or Zr-incorporated SBA-15 materials. J. Ind. Eng. Chem. 2017, 45, 257–265. [Google Scholar] [CrossRef]
- Sarihan, A.; Eren, E.; Eren, B.; Erdoğan, Y. Flux-enhanced polysulfone membranes blended with ABPBI as a novel additive. Mater. Today Commun. 2019, 20, 100594. [Google Scholar] [CrossRef]
- Xu, Z.L.; Qusay, F.A. Effect of polyethylene glycol molecular weights and concentrations on polyethersulfone hollow fiber ultrafiltration membranes. J. Appl. Polym. Sci. 2004, 91, 3398–3407. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular size distribution in three dimensional polymers. VI. Branched polymers containing A-R-Bf-1 type units. J. Am. Chem. Soc. 1952, 74, 2718–2723. [Google Scholar] [CrossRef]
- Jena, K.K.; Raju, K.V.S.N.; Prathab, B.; Aminabhavi, T.M. Hyperbranched polyesters: synthesis, characterization, and molecular simulations. J. Phys. Chem. B 2007, 111, 8801–8811. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhu, B.K.; Ma, X.T.; Xu, Y.Y. Porous membranes modified by hyperbranched polymers: I. Preparation and characterization of PVDF membrane using hyperbranched polyglycerol as additive. J. Membr. Sci. 2007, 290, 222–229. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Qian, Y.L.; Pang, D.X.; Zhu, B.K.; Xu, Y.Y. Porous membranes modified by hyperbranched polymers II.: Effect of the arm length of amphiphilic hyperbranched-star polymers on the hydrophilicity and protein resistance of poly(vinylidene fluoride) membranes. J. Membr. Sci. 2007, 304, 138–147. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Xu, Y.Y.; Zhu, B.K. Effect of amphiphilic hyperbranched-star polymer on the structure and properties of PVDF based porous polymer electrolytes. Solid State Ion. 2009, 180, 1517–1524. [Google Scholar] [CrossRef]
- Zhao, L.B.; Xu, Z.L.; Liu, M.; Wei, Y.M. Preparation and characterization of PSf hollow fiber membrane from PSf–HBPE–PEG400–NMP dope solution. J. Membr. Sci. 2014, 454, 184–192. [Google Scholar] [CrossRef]
- Sun, D.; Yang, P.; Sun, H.L.; Li, B.B. Preparation and characterization of cross-linked poly (vinyl alcohol)/hyperbranched polyester membrane for the pervaporation dehydration of ethylene glycol solution. Eur. Polym. J. 2015, 62, 155–166. [Google Scholar] [CrossRef]
- Zhao, L.B.; Liu, M.; Xu, Z.L.; Wei, Y.M.; Xu, M.X.; Jiang, B.H. Modification of polysulfone hollow fiber ultrafiltration membranes using hyperbranched polyesters with different molecular weights. Polym. Adv. Technol. 2015, 26, 353–361. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural characterization of thin-film polyamide reverse osmosis membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.H.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.H.; Tsuru, T. Application of interfacially polymerized polyamide composite membranes to isopropanol dehydration: Effect of membrane pre-treatment and temperature. J. Membr. Sci. 2014, 453, 384–393. [Google Scholar] [CrossRef]
- Liu, S.H.; Liu, M.; Xu, Z.L.; Wei, Y.M. A polyethersulfone–bisphenol sulfuric acid hollow fiber ultrafiltration membrane fabricated by a reverse thermally induced phase separation process. RSC Adv. 2018, 8, 7800–7809. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Liu, M.; Xu, Z.L.; Wei, Y.M.; Guo, X. A novel PES-TiO2 hollow fiber hybrid membrane prepared via sol-gel process assisted reverse thermally induced phase separation (RTIPS) method. J. Membr. Sci. 2017, 528, 303–315. [Google Scholar] [CrossRef]
- Yang, Q.; Chung, T.S.; Santoso, Y.E. Tailoring pore size and pore size distribution of kidney dialysis hollow fiber membranes via dual-bath coagulation approach. J. Membr. Sci. 2007, 290, 153–163. [Google Scholar] [CrossRef]
- Wang, K.Y.; Matsuura, T.; Chung, T.S.; Guo, W.F. The effects of flow angle and shear rate within the spinneret on the separation performance of poly(ethersulfone) (PES) ultrafiltration hollow fiber membranes. J. Membr. Sci. 2004, 240, 67–79. [Google Scholar] [CrossRef]
- Youm, K.H.; Kim, W.S. Prediction of intrinsic pore properties of ultrafiltration membrane by solute rejection curves: Effects of operating conditions on pore properties. J. Chem. Eng. Jpn. 1991, 24, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Michaels, A.S. Analysis and prediction of sieving curves for ultrafiltration membranes: A universal correlation ? Sep. Sci. Technol. 1980, 15, 1305–1322. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes. Desalination 2011, 272, 51–58. [Google Scholar] [CrossRef]
- Kwong, M.; Abdelrasoul, A.; Doan, H. Controlling polysulfone (PSF) fiber diameter and membrane morphology for an enhanced ultrafiltration performance using heat treatment. Results Mater. 2019, 2, 100021. [Google Scholar] [CrossRef]
- Lavanya, C.; Balakrishna, R.G. Naturally derived polysaccharides-modified PSF membranes: A potency in enriching the antifouling nature of membranes. Sep. Purif. Technol. 2020, 230, 115887. [Google Scholar] [CrossRef]










| Membrane Number | Casting Solution Compositions (wt %) | HBPE Code Name | |||
|---|---|---|---|---|---|
| PSf | HBPE | PEG400 | DMAc | ||
| MP0 | 18.0 | 0 | 18.7 | 62.3 | |
| MP1 | 18.0 | 1.0 | 18.7 | 62.3 | SP-1 |
| MP2 | 18.0 | 1.0 | 18.7 | 62.3 | SP-2 |
| MP3 | 18.0 | 1.0 | 18.7 | 62.3 | SP-3 |
| MP4 | 18.0 | 1.0 | 18.7 | 62.3 | SP-4 |
| SP-1 | 2160 | 1910 | 1.13 |
| SP-2 | 2660 | 2370 | 1.12 |
| SP-3 | 4150 | 3490 | 1.19 |
| SP-4 | 5840 | 4570 | 1.27 |
| Membranes | (nm) | MWCO (Da) |
|---|---|---|
| MP0 | 6.78 | 36,100 |
| MP1 | 3.43 | 11,600 |
| MP2 | 5.87 | 21,700 |
| MP3 | 5.68 | 26,100 |
| MP4 | 4.22 | 14,400 |
| Membrane | Preparation Method | Filler Loading | Breaking Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | Ref. |
|---|---|---|---|---|---|---|
| PSf | NIPS | 1.0 wt %HBPEs-PER ( = 2160) | 6.6 | 62.7 | 110.3 | This work |
| PSf | NIPS | 1.0 wt %HBPEs-PER ( = 11,200) | 6.2 | 73.5 | 107.0 | [23] |
| PSf | NIPS | 1.0 wt %HBPEs-TMP ( = 2470) | 6.1 | 84.4 | 89.1 | [25] |
| PSf | Heat treatment | Tension heating for 1 h at 185 °C | 7.31 | - | - | [36] |
| PSf | Heat treatment | Tension heating for 1 h at 195 °C | 5.51 | - | - | [36] |
| PSf | DIPS | - | 5.5 | 6.9 | - | [37] |
| PSf | DIPS | 1.0 wt % LGC | 8.7 | 10.3 | - | [37] |
| PSf | DIPS | 1.0 wt % LGC and 0.75 wt % caramel | 10.8 | 13.4 | - | [37] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhao, L.-B.; Yu, L.-Y.; Wei, Y.-M.; Xu, Z.-L. Structure and Properties of PSf Hollow Fiber Membranes with Different Molecular Weight Hyperbranched Polyester Using Pentaerythritol as Core. Polymers 2020, 12, 383. https://doi.org/10.3390/polym12020383
Liu M, Zhao L-B, Yu L-Y, Wei Y-M, Xu Z-L. Structure and Properties of PSf Hollow Fiber Membranes with Different Molecular Weight Hyperbranched Polyester Using Pentaerythritol as Core. Polymers. 2020; 12(2):383. https://doi.org/10.3390/polym12020383
Chicago/Turabian StyleLiu, Min, Long-Bao Zhao, Li-Yun Yu, Yong-Ming Wei, and Zhen-Liang Xu. 2020. "Structure and Properties of PSf Hollow Fiber Membranes with Different Molecular Weight Hyperbranched Polyester Using Pentaerythritol as Core" Polymers 12, no. 2: 383. https://doi.org/10.3390/polym12020383