The Potential of Polyethylene Terephthalate Glycol as Biomaterial for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
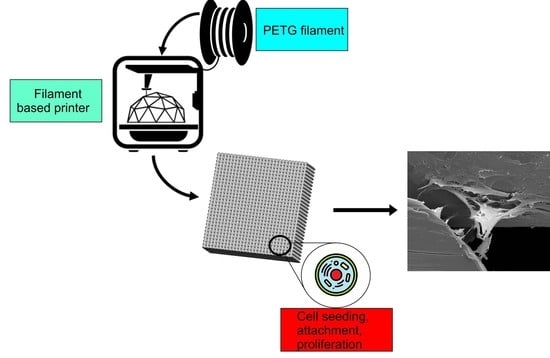
2.2. Scaffolds’ Fabrication
2.3. Morphological Analysis
2.4. Mechanical Compression Tests
2.5. Biological Analysis
2.5.1. Cell Culture
2.5.2. Alamar Blue Assay
2.5.3. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis
3.2. Scaffold Morphology
3.3. Mechanical Compression Test
3.4. Biological Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tayebi, L.; Moharamzadeh, K. Biomaterials for Oral and Dental Tissue Engineering; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Orlando, G. Regenerative Medicine Applications in Organ Transplantation; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Dwivedi, R.; Mehrotra, D. 3D bioprinting and craniofacial regeneration. J. Oral Biol. Craniofacial Res. 2020, 10, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bartolo, P.J.; Granja, P.L. A Single-Component Hydrogel Bioink for Bioprinting of Bioengineered 3D Constructs for Dermal Tissue Engineering. Mater. Horiz. 2018, 5, 1100–1111. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. 3D photo-fabrication for tissue engineering and drug delivery. Engineering 2015, 1, 90–112. [Google Scholar] [CrossRef] [Green Version]
- Christy, P.N.; Basha, S.K.; Kumari, V.S.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications–A review. J. Drug Deliv. Sci. Technol. 2020, 55, 101452. [Google Scholar] [CrossRef]
- Ghorbani, F.; Li, D.; Ni, S.; Zhou, Y.; Yu, B. 3D printing of acellular scaffolds for bone defect regeneration: A review. Mater. Today Commun. 2020, 22, 100979. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2019, 10, 6. [Google Scholar] [CrossRef]
- Baptista, R.; Guedes, M. Morphological and mechanical characterization of 3D printed PLA scaffolds with controlled porosity for trabecular bone tissue replacement. Mater. Sci. Eng. C 2020, 118, 111528. [Google Scholar] [CrossRef]
- Bártolo, P.; Chua, C.K.; Almeida, H.A.; Chou, S.M.; Lim, A.S.C. Biomanufacturing for tissue engineering: Present and future trends. Virtual Phys. Prototyp. 2009, 4, 203–216. [Google Scholar] [CrossRef]
- Melchels, F.P.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef] [Green Version]
- Bartolo, P.; Kruth, J.P.; Silva, J.; Levy, G.; Malshe, A.; Rajurkar, K.; Mitsuishi, M.; Ciurana, J.; Leu, M. Biomedical production of implants by additive electro-chemical and physical processes. CIRP Ann. 2012, 61, 635–655. [Google Scholar] [CrossRef]
- Hench, L.; Jones, J. Biomaterials, Artificial Organs and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Remes, A.; Williams, D. Immune response in biocompatibility. In The Biomaterials: Silver Jubilee Compendium; Elsevier: Oxford, UK, 1992; pp. 79–91. [Google Scholar]
- Bártolo, P.; Bidanda, B. Biomaterials and Prototyping Applications in Medicine; Springer: New York, NY, USA, 2008. [Google Scholar]
- Zhou, M.; Yang, X.; Li, S.; Kapat, K.; Guo, K.; Perera, F.H.; Qian, L.; Miranda, P.; Che, Y. Bioinspired channeled, rhBMP-2-coated β-TCP scaffolds with embedded autologous vascular bundles for increased vascularization and osteogenesis of prefabricated tissue-engineered bone. Mater. Sci. Eng. C 2020, 118, 111389. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; Wie, Y.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.M.P.; De Cássia Oliveira Paiva, N.; de Medeiros, R.V.B.; Pinto, M.C.X.; Tonelli, F.C.P.; Resende, R.R. Tissue Engineering: The Use of Stem Cells in Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Ribas, R.G.; Schatkoski, V.M.; Do Amaral Montanheiro, T.L.; De Menezes, B.R.C.; Stegemann, C.; Leite, D.M.G.; Thim, G.P. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: A review. Ceram. Int. 2019, 45, 21051–21061. [Google Scholar] [CrossRef]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.J.; Diver, C.; Bartolo, P. Polymer-ceramic composite scaffolds: The effect of hydroxyapatite and β-tri-calcium phosphate. Materials 2018, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Junior, J.R.L.; Nalesso, P.R.L.; Musson, D.; Cornish, J.; Fernanda, M.; Caetano, G.F.; Bartolo, P. Engineered 3D printed poly (ɛ-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 759–770. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Z.; Li, J.; Bartolo, P. Engineering the biological performance of hierarchical nanostructured poly(ε-carpolactone) scaffolds for bone tissue engineering. CIRP Ann. 2020, 69, 217–220. [Google Scholar] [CrossRef]
- Vyas, C.; Ates, G.; Aslan, E.; Harl, J.; Huang, B.; Bartolo, P. Three-Dimensional Printing and Electrospinning Dual-Scale Polycaprolactone Scaffolds with Low-Density and Oriented Fibers to Promote Cell Alignment. 3D Print. Addit. Manuf. 2020, 7, 105–113. [Google Scholar] [CrossRef]
- Liu, F.; Wang, W.; Mirihanage, W.; Hinduja, S.; Bartolo, P.J. A plasma-assisted bioextrusion system for tissue engineering. CIRP Ann. 2018, 67, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Caetano, G.F.; Wang, W.; Chiang, W.H.; Cooper, G.; Diver, C.; Blaker, J.J.; Frade, M.A.; Bartolo, P. 3D-Printed poly (ɛ-caprolactone)/graphene scaffolds activated with P1-latex protein for bone regeneration. 3D Print. Addit. Manuf. 2018, 5, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Caetano, G.; Violante, R.; Sant’Ana, A.B.; Murashima, A.B.; Domigos, M.; Gibson, A.; Bartolo, P.; Frade, M.A. Cellularized versus decellularized scaffolds for bone regeneration. Mater. Lett. 2016, 182, 318–322. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Vyas, C.; Byun, J.J.; El-Newehy, M.; Huang, Z.; Bartolo, P. Aligned multi-walled carbon nanotubes with nanohydroxyapatite in a 3D printed polycaprolactone scaffold stimulates osteogenic differentiation. Mater. Sci. Eng. C 2020, 108, 110374. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, T.; Yang, S.T.; Kniss, D.A. Thermal compression and characterization of three-dimensional nonwoven PET matrices as tissue engineering scaffolds. Biomaterials 2001, 22, 609–618. [Google Scholar] [CrossRef]
- Taniguchi, I.; Yoshida, S.; Hiraga, K.; Miyamoto, K.; Kimura, Y.; Oda, K. Biodegradation of PET: Current Status and Application Aspects. ACS Catal. 2019, 9, 4089–4105. [Google Scholar] [CrossRef]
- Montaudo, G.; Samperi, F.; Montaudo, M.S. Characterization of synthetic polymers by MALDI-MS. Prog. Polym. Sci. 2006, 31, 277–357. [Google Scholar] [CrossRef]
- Wu, T.; Hu, H.L.; Du, Y.P.; Jiang, D.; Yu, B.H. Discrimination of thermoplastic polyesters by MALDI-TOF MS and Py-GC/MS. Int. J. Polym. Anal. Charact. 2014, 19, 441–452. [Google Scholar] [CrossRef]
- Li, L. MALDI-MS for Polymer Characterization; MALDI MS; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Peacock, P.M.; McEwen, C.N. Mass spectrometry of synthetic polymers. Anal. Chem. 2004, 76, 3417–3428. [Google Scholar] [CrossRef]
- Hassan, M.; Omar, A.M.; Daskalakis, E.; Liu, F.; Bartolo, P. Preliminary studies on the suitability of PETG for 4D printing applications. In Proceedings of the 7th International Conference of Materials and Manufacturing Engineering (ICMMEN 2020), Thessaloniki, Greece, 2–3 July 2020; EDP Sceinces: Les Ulis, France, 2020; p. 6. [Google Scholar]
- Turner, S.R. Development of amorphous copolyesters based on 1, 4-cyclohexanedimethanol. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5847–5852. [Google Scholar] [CrossRef]
- Thomson, R.C.; Yaszemski, M.J.; Powers, J.M.; Mikos, A.G. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J. Biomater. Sci. Polym. Ed. 1996, 7, 23–38. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef]
- Porter, B.D.; Oldham, J.B.; He, S.L.; Zobitz, M.E.; Payne, R.G.; An, K.N.; Currier, B.L.; Mikos, A.G.; Yaszemski, M.J. Mechanical properties of a biodegradable bone regeneration scaffold. J. Biomech. Eng. 2000, 122, 286–288. [Google Scholar]
- Lotz, J.C.; Gerhart, T.N.; Hayes, W.C. Mechanical properties of trabecular bone from the proximal femur: A quantitative CT study. J. Comput. Assist. Tomogr. 1990, 14, 107–114. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]







| Parameters | Values |
|---|---|
| Layer thickness (mm) | 0.33 |
| Filament diameter (mm) | 0.35 |
| Nozzle temperature (°C) | 230 |
| Bed temperature (°C) | 60 |
| Printing speed (mm/s) | 20 |
| Compound | Molecular Formula | Molecular Weight (g/mol) |
|---|---|---|
| Terephthalic acid (TPA) | C8H6O4 | 166.13 |
| Ethylene Glycol (EG) | (CH2OH2) | 62.07 |
| Cyclohexanedimethanol (CHDM) | C8H16O2 | 144.21 |
| PCL | PETG-300 | PETG-350 | PETG-450 | |
|---|---|---|---|---|
| Compressive Modulus (MPa) | 76.1 ± 7.53 | 213± 12.2 | 196 ± 6.53 | 141 ± 8.20 |
| Compressive Strength (MPa) | 2.56 ± 0.26 | 5.44 ± 0.19 | 5.09 ± 0.21 | 3.55 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.H.; Omar, A.M.; Daskalakis, E.; Hou, Y.; Huang, B.; Strashnov, I.; Grieve, B.D.; Bártolo, P. The Potential of Polyethylene Terephthalate Glycol as Biomaterial for Bone Tissue Engineering. Polymers 2020, 12, 3045. https://doi.org/10.3390/polym12123045
Hassan MH, Omar AM, Daskalakis E, Hou Y, Huang B, Strashnov I, Grieve BD, Bártolo P. The Potential of Polyethylene Terephthalate Glycol as Biomaterial for Bone Tissue Engineering. Polymers. 2020; 12(12):3045. https://doi.org/10.3390/polym12123045
Chicago/Turabian StyleHassan, Mohamed H., Abdalla M. Omar, Evangelos Daskalakis, Yanhao Hou, Boyang Huang, Ilya Strashnov, Bruce D. Grieve, and Paulo Bártolo. 2020. "The Potential of Polyethylene Terephthalate Glycol as Biomaterial for Bone Tissue Engineering" Polymers 12, no. 12: 3045. https://doi.org/10.3390/polym12123045