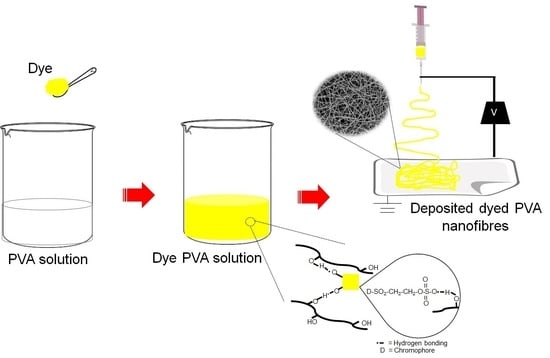
Dope-Dyeing of Polyvinyl Alcohol (PVA) Nanofibres with Remazol Yellow FG
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Dye Concentration on Fibre Diameter
3.2. Effect of Dye Concentration on Fibre Morphology and Colorimetric Properties of Dyed Fibres
3.3. FTIR ATR Analysis
3.4. TGA Analysis
3.5. UV Visible Spectroscopy Analysis
3.6. Flourescent Spectroscopy Analysis
3.7. Dye-Polymer Interaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. One-dimensional and two-dimensional synergized nanostructures for high-performing energy storage and conversion. InfoMat 2020, 2, 3–32. [Google Scholar] [CrossRef] [Green Version]
- Chinnappan, A.; Baskar, C.; Baskar, S.; Ratheesh, G.; Ramakrishna, S. An overview of electrospun nanofibers and their application in energy storage, sensors and wearable/flexible electronics. J. Mater Chem. C 2017, 5, 12657–12673. [Google Scholar] [CrossRef]
- Gorji, M.; Jeddi, A.; Gharehaghaji, A.A. Fabrication and characterization of polyurethane electrospun nanofiber membranes for protective clothing applications. J. Appl. Polym. Sci. 2012, 125, 4135–4141. [Google Scholar] [CrossRef]
- Najafi, M.; Frey, M.W. Electrospun Nanofibers for Chemical Separation. Nanomaterials 2020, 10, 982. [Google Scholar] [CrossRef]
- Baji, A.; Agarwal, K.; Oopath, S.V. Emerging Developments in the Use of Electrospun Fibers and Membranes for Protective Clothing Applications. Polymers 2020, 12, 492. [Google Scholar] [CrossRef] [Green Version]
- Sumin, L.; Kimura, D.; Yokoyama, A.; Lee, K.; Park, J.C.; Kim, I. The effects of laundering on the mechanical properties of mass-produced nanofiber web for use in wear. Text. Res. J. 2009, 79, 1085–1090. [Google Scholar] [CrossRef]
- Sumin, L.; Kimura, D.; Lee, K.H.; Park, J.C.; Kim, I.S. The effect of laundering on the thermal and water transfer properties of mass-produced laminated nanofiber web for use in wear. Text. Res. J. 2010, 80, 99–105. [Google Scholar] [CrossRef]
- Yoon, B.; Lee, S. Designing waterproof breathable materials based on electrospun nanofibers and assessing the performance characteristics. Fibers Polym. 2011, 12, 57–64. [Google Scholar] [CrossRef]
- Yan, X.; You, M.H.; Lou, T.; Yu, M.; Zhang, J.C.; Gong, M.G.; Lv, F.Y.; Huang, Y.Y.; Long, Y.Z. Colorful Hydrophobic Poly (Vinyl Butyral)/Cationic Dye Fibrous Membranes via a Colored Solution Electrospinning Process. Nanoscale Res. Lett. 2016, 11, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fantini, D.; Costa, L. Dye, fluorophores and pigment coloration of nanofibers produced by electrospinning. Polym. Adv. Technol. 2009, 20, 111–121. [Google Scholar] [CrossRef]
- Abbasipour, M.; Khajavi, R. Nanofiber bundles and yarns production by electrospinning: A review. Adv. Poly. Technol. 2013, 32, 1–9. [Google Scholar] [CrossRef]
- Becerir, B. Color concept in textiles: A review. J. Text. Eng. Fash. Technol. 2017, 1, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Khatri, M.; Ahmed, F.; Shaikh, I.; Phan, D.N.; Khan, Q.; Khatri, Z.; Lee, H.; Kim, I.S. Dyeing and characterization of regenerated cellulose nanofibers with vat dyes. Carbohydrate Polym. 2017, 174, 443–449. [Google Scholar] [CrossRef]
- Khatri, Z.; Arain, R.A.; Jatoi, A.W.; Mayakrishnan, G.; Wei, K.; Kim, I.S. Dyeing and characterization of cellulose nanofibers to improve color yields by dual padding method. Cellulose 2013, 20, 1469–1476. [Google Scholar] [CrossRef]
- Mehdi, M.; Mahar, F.K.; Qureshi, U.A.; Khatri, M.; Khatri, Z.; Ahmed, F.; Kim, I.S. Preparation of colored recycled polyethylene terephthalate nanofibers from waste bottles: Physicochemical studies. Adv. Polym. Technol. 2018, 37, 2820–2827. [Google Scholar] [CrossRef]
- Balakrishnan, N.K.; Koening, K.; Seide, G. The effect of dye and pigment concentrations on the diameter of melt-electrospun polylactic acid fibers. Polymer 2020, 12, 2321. [Google Scholar] [CrossRef]
- Khatri, A.; Ali, S.; Jhatial, A.K.; Kim, S.H. Dyeability of polyurethane nanofibres with disperse dyes. Color. Technol. 2015, 131, 374–378. [Google Scholar] [CrossRef]
- Daneshvar, E.; Amani Tehran, M.; Gorji Kandi, S.; Zeighami, F. Investigating the characteristics of two different methods in nanofiber yarn coloration. J. Text. Inst. 2016, 107, 833–841. [Google Scholar] [CrossRef]
- Zhang, Y.J. Research on the properties of dope-dyed bamboo pulp staple fibers. In IOP Conference Series. Mater. Sci. Eng. 2016, 137, 012022. [Google Scholar]
- Gurudatt, K.; De, P.; Rakshit, A.K.; Bardhan, M.K. Dope-dyed polyester fibers from recycled PET wastes for use in molded automotive carpets. J. Ind. Text. 2015, 34, 167–179. [Google Scholar] [CrossRef]
- Tazehkand, M.N. The investigation of anti-bacterial activity of Reactive Red-120. J. Pharmacol. Med. Chem. 2017, 2, 1–3. [Google Scholar]
- Li, L.; Jia, Z.; Peng, Y.; Godar, S.; Getov, I.; Teng, S.; Alper, J.; Alexov, E. Forces and Disease: Electrostatic force differences caused by mutations in kinesin motor domains can distinguish between disease-causing and non-disease-causing mutations. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adli, F.A. Study on the Formation of Dyed PVA Nanofibres Using Reactive Dye. Bachelor’s Thesis, Universiti Teknologi MARA, Shah Alam, Selangor, 2017. [Google Scholar]
- Sultana, S.; Fatema, U.K.; Islam, A. Sensitivity Analysis of Vynyl Sulphone and Bis-Monochlorotriazine Reactive Groups of Reactive Dyes. Eur. Sci. J. 2016, 12, 337–346. [Google Scholar]
- Rivlin, J. The Dyeing of Textile Fibers: Theory and Practice, 1st ed.; Joseph Rivlin Assoc.: Philadelphia, PA, USA, 1992; pp. 138–139. [Google Scholar]
- Qodri, A.A. Fotodegradasi Zat Warna Remazol Yellow FG Dengan Fotokatalis Komposit TiO2/SiO2. Bachelor’s Thesis, Universiti Sebelas Maret, Kota Surakarta, Indonesia, 2011. [Google Scholar]
- Rashad, M.; Kim, S.-S.; Huh, M.W.; Yoon, N.-S. Dyeing of polyvinyl alcohol fibers in filament yarn form with reactive and vat dyes. J. Korean Soc. Dyers Finish. 2007, 19, 17–22. [Google Scholar]
- Khatri, M.; Ahmed, F.; Jatoi, A.W.; Mahar, R.B.; Khatri, Z.; Kim, S.K. Ultrasonic dyeing of cellulose nanofibers. Ultrason. Sonochem. 2016, 31, 350–354. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Lin, T.; Wang, H.; Wang, H.; Wang, X. Charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology 2004, 15, 1375–1381. [Google Scholar] [CrossRef]
- Wang, L.; Topham, P.D.; Mykhaylyk, O.O.; Yu, H.; Ryan, A.J.; Fairclough, P.A.; Bras, W. Self-assembly-driven electrospinning: The transition from fibers to intact beaded morphologies. Macromolecular 2015, 36, 1437–1443. [Google Scholar] [CrossRef]
- Affandi, N.D.N.; Ahmad, M.R.; Saleh, S.H.; Remeli, M.F.; Teo, N.H.H.N.I.; Amran, N.F. A Study on the Formation of PVA/Kenaf Nanofibres via Electrospinning. Pertanika J. Sci. Technol. 2017, 25, 85–92. [Google Scholar]
- Feng, B.; Tu, H.; Yuan, H.; Peng, H.; Zhang, Y. Acetic-acid-mediated miscibility toward electrospinning homogeneous composite nanofibers of GT/PCL. Biomacromolecules 2012, 13, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Reneker, D.H. Droplet-jet shape parameters predict electrospun polymer nanofiber diameter. Polymer 2019, 168, 155–158. [Google Scholar] [CrossRef]
- Veerabhadraiah, A.; Ramakrishna, S.; Angadi, G.; Venkatram, M.; Ananthapadmanabha, V.K.; NarayanaRao, N.M.H.; Munishamaiah, K. Development of polyvinyl acetate thin films by electrospinning for sensor applications. Appl. Nanosci. 2017, 7, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Shivkumar, S. Molecular weight dependent structural regimes during the electrospinning of PVA. Matt. Lett. 2007, 61, 2325–2328. [Google Scholar] [CrossRef]
- Ding, Z.; Salim, A.; Ziaie, B. Selective nanofibers deposition through field—Enhanced electrospinning. Langmuir 2009, 25, 9648–9652. [Google Scholar] [CrossRef]
- Hann, R.A.; Beck, N.C. Dye Diffusion Thermal Transfer (D2T2) Color Printing. J. Imag. Technol. 1990, 16, 238–241. [Google Scholar]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar]
- Chen, C.K.; Liao, M.G.; Wu, Y.L.; Fang, Z.Y.; Chen, J.A. Preparation of Highly Swelling/Antibacterial Cross-Linked N-Maleoyl-Functional Chitosan/Polyethylene Oxide Nanofiber Meshes for Controlled Antibiotic Release. Mol. Pharm. 2020, 17, 3461–3476. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Zhu, T.; Gao, S.; Wei, B.; Bi, L. Identification of tartrazine and sunset yellow by fluorescence spectroscopy combined with radial basis function neural network. Chin. Opt. Lett. 2009, 7, 1058–1060. [Google Scholar] [CrossRef]
- Deniz, E.; Sortino, S.; Raymo, F.M. Fluorescence switching with a photochromic auxochrome. J. Phys. Chem. Lett. 2010, 1, 3506–3509. [Google Scholar] [CrossRef]
- Slark, A.T.; Hadgett, P.M. The effect of polymer structure on specific interactions between dye solutes and polymers. Polymer 1999, 40, 1325–1332. [Google Scholar] [CrossRef]
- Briscoe, B.; Luckham, P.; Zhu, S. The effects of hydrogen bonding upon the viscosity of aqueous poly(vinyl alcohol) solutions. Polymer 2000, 41, 3851–3860. [Google Scholar] [CrossRef]










| Dye Content (wt.%) | L* | a* | b* |
|---|---|---|---|
| 2.5 | 6.05 | 1.23 | 10.28 |
| 5.0 | 6.39 | 3.74 | 10.78 |
| 7.5 | 5.02 | 5.21 | 8.62 |
| 10.0 | 11.68 | 9.85 | 20.03 |
| 12.5 | 9.79 | 7.98 | 16.77 |
| Dye Content (wt.%) | L* | a* | b* |
|---|---|---|---|
| 0.2 | 0.37 | 1.54 | 0.64 |
| 0.4 | 0.31 | 1.32 | 0.54 |
| 0.6 | 0.28 | 1.25 | 0.48 |
| 0.8 | 0.19 | 1.04 | 0.33 |
| 1.0 | 0.03 | 0.18 | 0.05 |
| Sample | Remazol Yellow FG |
|---|---|
| Max Emission wavelength (nm) | 530 |
| Fluorescence peak (nm) | 484, 530 |
| Fluorescence spectra width (nm) | 450–600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadil, F.; Adli, F.A.; Affandi, N.D.N.; Harun, A.M.; Alam, M.K. Dope-Dyeing of Polyvinyl Alcohol (PVA) Nanofibres with Remazol Yellow FG. Polymers 2020, 12, 3043. https://doi.org/10.3390/polym12123043
Fadil F, Adli FA, Affandi NDN, Harun AM, Alam MK. Dope-Dyeing of Polyvinyl Alcohol (PVA) Nanofibres with Remazol Yellow FG. Polymers. 2020; 12(12):3043. https://doi.org/10.3390/polym12123043
Chicago/Turabian StyleFadil, Fatirah, Farah Atiqah Adli, Nor Dalila Nor Affandi, Ahmad Mukifza Harun, and Mohammad Khursheed Alam. 2020. "Dope-Dyeing of Polyvinyl Alcohol (PVA) Nanofibres with Remazol Yellow FG" Polymers 12, no. 12: 3043. https://doi.org/10.3390/polym12123043