Elucidation of Antimicrobial Silver Sulfadiazine (SSD) Blend/Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) Immobilised with Collagen Peptide as Potential Biomaterial
Abstract
:1. Introduction
2. Materials and Methods
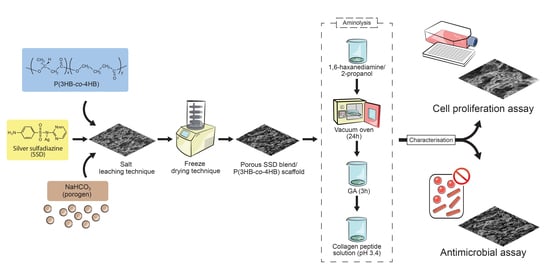
2.1. Fabrication of Porous SSD Blend/P(3HB-co-4HB) Aminolysed Collagen Peptide Scaffolds
2.2. Characterization of Porous SSD Blend /P(3HB-co-4HB)-Collagen Peptide
2.3. Antimicrobial Assay
2.4. Cell Proliferation Assay
2.5. Statistical Analysis
3. Results and Discussions
3.1. SSD Blend/P(3HB-co-4HB) Aminolysed Collagen Peptide
3.2. Hydrophilicity of SSD Blend/P(3HB-co-4HB)-Collagen Peptide Scaffold Wettability
3.3. Biocompatibility and Cell Proliferation of SSD Blend/P(3HB-co-4HB) Aminolysed Collagen Peptide Scaffolds
3.4. Antimicrobial Analysis of Fabricated SSD Blend/P(3HB-co-4HB) Aminolysed Collagen Peptide Scaffold.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Özkale, B.; Sakar, M.S.; Mooney, D.J. Active biomaterials for mechanobiology. Biomaterials 2020, 267, 120497. [Google Scholar] [CrossRef]
- Rahmati, M.; Silva, E.; Reseland, J.E.; Heyward, C.A.; Haugen, H.J. Biological responses to physicochemical properties of biomaterial surface. Chem. Soc. Rev. 2020, 49, 5178–5224. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.A.; Petzold, C.; Pluduma-LaFarge, L.; Kumermanis, M.; Haugen, H.J. Structural and Chemical Hierarchy in Hydroxyapatite Coatings. Materials 2020, 13, 4447. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, J.M.; Grainger, D.D. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Vladkova, T.G. Surface Engineered Polymeric Biomaterials with Improved Biocontact Properties. Int. J. Polym. Sci. 2010, 2010, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Vigneswari, S.; Chai, J.M.; Shantini, K.; Bhubalan, K.; Amirul, A.A. Designing Novel Interfaces via Surface Functionalization of Short-Chain-Length Polyhydroxyalkanoates. Adv. Polym. Technol. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Members 2018, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Huong, K.-H.; Teh, C.-H.; Amirul, A.A. Microbial-based synthesis of highly elastomeric biodegradable poly(3-hydroxybutyrate- co -4-hydroxybutyrate) thermoplastic. Int. J. Biol. Macromol. 2017, 101, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Vigneswari, S.; Chai, J.M.; Kamarudin, K.H.; Amirul, A.-A.A.; Focarete, M.L.; Ramakrishna, S. Elucidating the Surface Functionality of Biomimetic RGD Peptides Immobilized on Nano-P(3HB-co-4HB) for H9c2 Myoblast Cell Proliferation. Front. Bioeng. Biotechnol. 2020, 8, 567693. [Google Scholar] [CrossRef] [PubMed]
- Vigneswari, S.; Murugaiyah, V.; Kaur, G.; bdul Khalil, H.P.; Amirul, A.A. Biomacromolecule immobilization: Grafting of fish-scale collagen peptides onto aminolyzed P(3HB- co -4HB) scaffolds as a potential wound dressing. Biomed. Mater. 2016, 11, 055009. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ke, Y.; Ren, L.; Wu, G.; Chen, X.; Zhao, Q. Surface engineering of PHBV by covalent collagen immobilization to improve cell compatibility. J. Biomed. Mater. Res. Part A 2008, 88, 616–627. [Google Scholar] [CrossRef]
- Chai, C.; Amirul, A.; Vigneswari, S. Data on the effect of electrospinning parameters on the morphology of the nanofibrous poly(3-hydroxybutyrate-co-4-hydroxybutyrate) scaffolds. Data Brief 2020, 28, 104777. [Google Scholar] [CrossRef]
- Ismail, I.; Gurusamy, T.P.; Ramachandran, H.; Amirul, A.A.-A. Enhanced production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) copolymer and antimicrobial yellow pigmentation from Cupriavidus sp. USMAHM13 with antibiofilm capability. Prep. Biochem. Biotechnol. 2017, 47, 388–396. [Google Scholar] [CrossRef]
- Vigneswari, S.; Khalil, H.P.S.A.; Amirul, A.A. Designing of Collagen Based Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Scaffolds for Tissue Engineering. Int. J. Polym. Sci. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Qi, Y.; Pan, Z.; He, Y.; Liu, X.; Cui, S.; Ding, J. Design and preparation of quasi-spherical salt particles as water-soluble porogens to fabricate hydrophobic porous scaffolds for tissue engineering and tissue regeneration. Mater. Chem. Front. 2018, 2, 1539–1553. [Google Scholar] [CrossRef]
- Subia, B.; Kundu, J.; Kundu, C.S. Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications. Tissue Eng. 2010, 141. [Google Scholar] [CrossRef]
- Siegler, J.C.; Midgley, A.W.; Polman, R.; Lever, R. Effects of Various Sodium Bicarbonate Loading Protocols on the Time-Dependent Extracellular Buffering Profile. J. Strength Cond. Res. 2010, 24, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Lutzweiler, G.; Halili, A.N.; Vrana, N.E. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceuticals 2020, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Oikonomopoulos, I.; Perraki, T.; Tougiannidis, N. FTIR Study of Two Different Lignite Lithotypes From Neocene Achlada Lignite Deposits In Nw Greece. Bull. Geol. Soc. Greece 2017, 43, 2284–2293. [Google Scholar] [CrossRef]
- El-Feky, G.S.; El-Banna, S.T.; El-Bahy, G.; Abdelrazek, E.; Kamal, M. Alginate coated chitosan nanogel for the controlled topical delivery of Silver sulfadiazine. Carbohydr. Polym. 2017, 177, 194–202. [Google Scholar] [CrossRef]
- Ratner, B.D. A pore way to heal and regenerate: 21st century thinking on biocompatibility. Regen. Biomater. 2016, 3, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Ramos, T.A.D.S.; Damanik, F.; Le, B.Q.; Wieringa, P.; Bennink, M.L.; Van Blitterswijk, C.; De Boer, J.; Moroni, L. A combinatorial approach towards the design of nanofibrous scaffolds for chondrogenesis. Sci. Rep. 2015, 5, 14804. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Liu, S.; Ma, Y.; Xu, W.; Meng, W.; Lin, X.; Wang, W.; Wang, S.; Zhang, J. Innovative biodegradable poly(L-lactide)/collagen/ hydroxyapatite composite fibrous scaffolds promote osteoblastic proliferation and differentiation. Int. J. Nanomed. 2017, 12, 7577–7588. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Jiang, Y.; Hu, J. Collagen incorporation into waterborne polyurethane improves breathability, mechanical property, and self-healing ability. Compos. Part A: Appl. Sci. Manuf. 2020, 133, 105854. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014, 9, 2335–2344. [Google Scholar] [CrossRef] [Green Version]
- Toledo, A.L.M.M.; Ramalho, B.S.; Picciani, P.H.S.; Baptista, L.; Martinez, A.M.B.; Dias, M.L. Effect of three different amines on the surface properties of electrospun polycaprolactone mats. Int. J. Polym. Mater. 2020, 1–13. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface Modification of Polycaprolactone Membrane via Aminolysis and Biomacromolecule Immobilization for Promoting Cytocompatibility of Human Endothelial Cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Solouk, A.; Mirzadeh, H.; Shokrgozar, M.A.; Solati-Hashjin, M.; Najarian, S.; Seifalian, A.M. The Study of Collagen Immobilization on a Novel Nanocomposite to Enhance Cell Adhesion and Growth. Iran. Biomed. J. 2011, 15, 6–14. [Google Scholar] [PubMed]
- Chen, Q.; Bruyneel, A.A.N.; Carr, C.; Czernuszka, J. Bio-mechanical Properties of Novel Bi-layer Collagen-Elastin Scaffolds for Heart Valve Tissue Engineering. Procedia Eng. 2013, 59, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Bakare, R.A.; Bhan, C.; Raghavan, D. Synthesis and characterization of collagen grafted poly(hydroxybutyrate-co-valerate) (PHBV) scaffold for loading of bovine serum albumin capped silver (Ag/BSA) nanoparticles in the potential use of tissue engineering application. Biomacromolecules 2014, 15, 423–435. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef]









| Polymer | Amount of Collagen Retained (%) |
|---|---|
| P(3HB-co-4HB) + 2.5 wt% collagen | 78 ± 2 a |
| P(3HB-co-4HB) + 5 wt% collagen | 80 ± 3 a,b |
| P(3HB-co-4HB) + 7.5 wt% collagen | 82 ± 3 a,b |
| P(3HB-co-4HB) + 10 wt% collagen | 83 ± 2 a,b |
| P(3HB-co-4HB) + 12.5 wt% collagen | 85 ± 1 b |
| Biopolymer/Materials | Water Contact Angle Results | References |
|---|---|---|
| PLGA/collagen nanofibers | It is recorded that the contact angle of this electrospun PLGA/collagen scaffold is 70° and the addition of collagen increased the wettability of the PLGA to be developed as tissue engineering scaffolds for regenerative medicine | [26] |
| PLLA/collagen nanofibers | The incorporation of collagen to the PLLA biopolymer nanofibers to improve the surface wettability resulted in a water contact angle of 80° for the development of bone graft. | [27] |
| Polyurethane/collagen by green facile method | The addition of collagen to the polyurethane exhibited water contact angle between 80–85° for the development of smart materials that are environmentally friendly and easier to process | [28] |
| collagen/PLCL nanofibers | The PLCL electrospun nanofiber with collagen had an increased wettability between 0° (10 s) and 24° (5 s) for the development of desirable scaffold for | [29] |
| P(3HB-co-4HB) aminolysed collagen peptide | The porous P(3HB-co-4HB) scaffolds incorporated with collagen in this study showed an increased surface wettability with water contact between 4.3° ± 1.8° − 43.7° ± 1.7° which can be further developed as tissue regenerative scaffolds | Current paper |
| Time (h) | Inhibition of Microorganisms (%) | ||
|---|---|---|---|
| Staphylococus auerus ATCC 12600 | Pseudomonas aeruginosa ATCC 17588 | Candida albicans | |
| 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 6 | 24 ± 5 | 25 ± 7 | 32 ± 2 |
| 12 | 45 ± 3 | 55 ± 7 | 61 ± 8 |
| 24 | 65 ± 9 | 92 ± 5 | 82 ± 8 |
| 48 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigneswari, S.; Gurusamy, T.P.; Abdul Khalil, H.P.S.; Ramakrishna, S.; Amirul, A.-A.A. Elucidation of Antimicrobial Silver Sulfadiazine (SSD) Blend/Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) Immobilised with Collagen Peptide as Potential Biomaterial. Polymers 2020, 12, 2979. https://doi.org/10.3390/polym12122979
Vigneswari S, Gurusamy TP, Abdul Khalil HPS, Ramakrishna S, Amirul A-AA. Elucidation of Antimicrobial Silver Sulfadiazine (SSD) Blend/Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) Immobilised with Collagen Peptide as Potential Biomaterial. Polymers. 2020; 12(12):2979. https://doi.org/10.3390/polym12122979
Chicago/Turabian StyleVigneswari, Sevakumaran, Tana Poorani Gurusamy, H. P. S. Abdul Khalil, Seeram Ramakrishna, and Al-Ashraf Abdullah Amirul. 2020. "Elucidation of Antimicrobial Silver Sulfadiazine (SSD) Blend/Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) Immobilised with Collagen Peptide as Potential Biomaterial" Polymers 12, no. 12: 2979. https://doi.org/10.3390/polym12122979