4.1. Glass Transition Temperature
Glass transition temperature (
Tg) may be seen as one criterion of the miscibility of a polymer blend from a macroscopic point of view. The blend is seen as a single-phase polymer blend (miscible blend) when it exhibits a single and composition-dependent
Tg. Immiscibility can be concluded when two
Tgs that are approximately close to the
Tg of parent polymers are observed. The Fox equation as in Equation (3) is commonly referred to for the evaluation or prediction of the
Tg values of miscible binary blends.
where
WA,
Tg,
A and
WB,
Tg,
B represent the mass fraction and
Tg of the respective polymer. The dashed curve in
Figure 1b is calculated after Equation (3). The entire composition of semi-crystalline/amorphous PEO/PMA blends in this study exhibit only a single and composition-dependent
Tg. The heating cycle of DSC thermograms are displayed in
Figure 1a. Moreover, the experimental
Tg values are in good agreement with the
Tgs predicted after the Fox equation. This may suggest that the polymer pair is miscible and homogeneous in the amorphous phase as well as in the molten state under this experimental condition for all compositions.
Figure 2a displays that the
Tg of PMA decreases with elevating salt content (
WS). Unlike PMA,
Tg of neat PEO elevates with increasing
WS. This phenomenon implies that the salt may be more soluble in PEO than PMA. It is interesting to note that the miscible PEO/PMA blends act differently with the addition of salt as shown in
Figure 2. The blends with
WPEO ≥ 0.7 after the addition of salt
WS still show single-composition-dependent
Tg for all studied salt concentrations (
WS = 0–0.17), which may indicate miscibility of the ternary mixtures in the amorphous phase and in the molten state. PEO
80 displays the highest
Tg values at
WS = 0–0.09 as compared to other systems
WPEO ≥ 0.7 with the same amount of salt. It implies PEO
80 may be an effective host for LiClO
4 for the enhancement of the ionic conductivity. Normally, the increase in
Tg indicates the stiffness of the polymer chains at an increasing temperature. In this case, the stiffness of the chain segments comes from the interaction of salt molecules and polymer chains. However, the increase in
Tg is only limited in the range of low salt content, as it increases up to saturation of the mixture due to the solubility limit of salt in the respective polymers [
23,
24]. When
WPEO ≤ 0.6 at
WS ≥ 0.05, the heterogeneity in the melt is inferred (i.e., liquid–liquid phase separation). It is deduced by the presence of two
Tgs that correspond to the parent polymers. From
Figure 2b, another observation on the salt localization in the PEO phase more than PMA can be seen. In the immiscible systems, the
Tgs of PEO are constantly observed at slightly higher values than the neat PEO, whereas the
Tgs of PMA lie closely to that of the neat PMA.
These
Tg findings are in good agreement with the descriptions in the Introduction. Macroscopically, we observe situation (I) in the system with
WPEO ≤ 0.6 at
WS ≥ 0.07, and situation (II) in the system of
WPEO ≥ 0.7 at
WS ≤ 0.10. One may also observe the morphology of situation (III) in binary PEO–salt systems (
WPEO = 1) at
WS ≥ 0.10 or beyond the salt saturation of the mixture. This is confirmed by the
Tg of the respective mixture, which is closed to
Tg of neat PEO. Furthermore, the precipitation of Li–salt was observed from optical inspection. The mixture with morphology (III) can be differentiated into salt-rich or salt-poor phases [
21]. The salt-rich phase (phase″) is mainly the pure salt phase, and salt-poor phase (phase’) is the phase of highly diluted salt solution in the polymer phase. The salt content of salt-poor phase (
WS’) can be elucidated instantly from the plot of
Tg against
WS. Hence, the preferential localization of salt in the different polymer phases of the immiscible ternary mixture can be detected based on their respective
Tg values. For instance, the PEO
50 system added with
WS at 0.091 (black arrow in
Figure 2b) is seen to contain approximately 1 wt.% of Li–salt dispersed in the PEO phase as the
Tg of PEO in PEO
50 is equivalent to
Tg of PEO at
WS = 0.01 (black arrow in
Figure 2a).
These ternary mixtures are miscible in the molten state and in the amorphous state under this experimental condition at sufficiently low salt content for all blend compositions. The mixtures are also miscible in molten state and in the amorphous phase at high salt content only when the PEO content is in excess, WPEO ≥ 0.7. The liquid–liquid phase separation in the molten state may be deduced when the PEO content is WPEO ≤ 0.6 at high salt content, WS ≥ 0.05. The mixtures under discussion are comprised semi-crystalline/amorphous PEO/PMA SPEs. Hence, at 25 °C, the SPEs (which is true either for miscible or liquid–liquid phase-separated systems in the melt) are seen as liquid–solid phase separated systems due to the crystallization of PEO, which takes place upon cooling from the melt. The morphological studies of these SPEs that will be discussed in the subsequent section were carried out at temperatures T = 80 °C and T = 25 °C using optical microscopy (OM) with the aim of elucidating the phase behavior of the mixture in the melt and in the liquid–solid form from the microscopic level aside from distinguishing the correlation between Tg and morphology. However, the correlation between the electrical properties and miscibility will not be attempted because only liquid–solid phase-separated systems at 25 °C were studied in all cases. Instead, preferential localization of salt in different polymer phases and the percolation path of the systems will be correlated to the relaxation and conductance of the systems.
From
Figure 2a, we may also discuss the variation of
Tg of the polymer blends that are still miscible in the molten and amorphous state after the addition of salt, for instance,
WPEO ≥ 0.7, in terms of thermodynamics based on the empirical observation [
21]. The quantity of
Tg, and its Δ
CP can be correlated as follows
Δ
Sglass denotes the change in entropy of the glassy state after addition of salts that indirectly describes the deviation of the glass from the equilibrium. Equation (4a) illustrates the Δ
CP serves as the factor of proportionality of –Δ
Sglass and
Tg/
Tgo. It is also true in the small range of
Tgo to
Tg that Δ
CP is assumed to be independent of temperature (this observation is only applicable for thermodynamically miscible system, i.e., PEO–PEO
70 and PMA). Approximately, the quantity of
Tg/
Tgo may also be expressed as below
where the slope of plot
Tg against
WS reads,
≡
Γ. The relationship of Equations (4) and (5) explains that the slope
Γ is interconnected to the partial molar entropy of the polymer pair in the ternary mixture that can be defined as
. The mean value of Δ
CP of the polymer pair with different salt content is employed for estimation of
on condition that the ternary mixtures lies in the linearity. Furthermore, the glass transition is not accompanied by the change in energy; hence, we may formulate the related chemical potential or molar partial Gibbs free energy by
The relationship of (4) and (5) shows when the slope
Γ is positive; the reduced
Tg/
Tgo of the mixture should be larger than unity and Δ
Sglass would be negative. The positive gradient,
Γ > 0 reflects the freezing in of degrees of freedom of the polymer chains, whereas a negative gradient,
Γ < 0 shows the increase in the chain’s degrees of freedom of the polymer. Experimentally, we observe that the salt affects the respective binary polymer-salt mixtures differently. PEO has a positive slope,
Γ > 0 with increasing
WS, whereas PMA has a negative slope,
Γ < 0 with increasing
WS. It suggests that the addition of salt in PEO leads to a higher stiffness of polymer chains whereas salt in PMA increases the flexibility of polymer chains. This discussion is also relevant for miscible ternary mixtures of PEO/PMA/LiClO
4. The gradient
Γ of
WPEO ≥ 0.7 (i.e., PEO
90 and PEO
70) is reduced gradually with the increasing content of PMA. This indicates that addition of PMA in the PEO–salt systems increases the degree of freedom of the polymer chains in the miscible polymer blends (increasing the chain flexibility). Besides, it is worth noting that addition of certain amount of PMA into the PEO–salt system also drives the systems closer to equilibrium, as reflected in the Δ
G. The corresponding parameters
Γ of miscible PEO/PMA/LiClO
4 mixtures are summarized in
Table 2.
4.2. Crystallinity
The degree of crystallinity (
X*) of PEO in the ternary mixtures was estimated from Equation (2). The melting enthalpy (Δ
Hm) of PEO was obtained from the area under the melting endotherm of the thermograms. Δ
Hm is an essential quantity for the estimation of the degree of crystallinity (
X*) for semi-crystalline polymer as the extent of the liquid–solid phase separation can be evaluated from crystallinity. The degree of crystallinity allows for rough estimation of the amorphous content. Hence, in principle, the reduction in crystallinity of the crystalline phase leads to an increase in the amorphous phase. It is widely accepted that ion percolation (conductivity) takes place only in the amorphous phase, thus, a rough estimation of the composition of the amorphous phase might be useful to understand electric conductivity. Equation (2) describes the degree of
X* of PEO, which is directly proportional to Δ
Hm of PEO, if the enthalpy of 100% crystallinity of PEO serves as the factor of proportionality
Figure 3 depicts the
X* of PEO in the ternary mixtures as a function of
WPEO. The dashed curve in the plot of
X* against
WPEO is the constancy curve of crystallinity of PEO in the ternary mixtures. The suppression of
X* from the constancy curve may be caused by the entrapment of the other component in the crystalline phase (in this case, PEO crystalline).
Figure 3 illustrates that the
X* of salt-free high molar mass PEO is 70% and starts to gradually level off to ~20% with increasing of salt content up to
WS = 0.167 for binary PEO–salt system. The deviation from the PEO crystallinity constancy curve is observed for all systems with different blend compositions and salt content.
When PEO crystallinity in both salt-free and salt-added systems is at low salt content, WS ≤ 0.05 displays no significant difference. The further suppression of X* of PEO is observed with higher content of salt, WS ≥ 0.07, which is true for all blends under discussion. This indicates that with small amounts of salt in the mixture, the PEO crystallinity of the semi-crystalline/amorphous mixture is not really affected by the presence of salt and the further suppression of X* only starts with increasing amounts of salt in the mixture. These observations are true for both miscible and immiscible systems of this study. The suppression of crystallinity is normally true for miscible systems, as the crystallization behavior of the crystalline component in the immiscible systems (or liquid–liquid phase separated systems in the melt) is expected to have close approximation to the neat crystalline polymer, and it is normally unaffected by the presence of other components. Nevertheless, from this study, we note that salt and PMA are fond of PEO. Hence, we may see the exclusion of salt and PMA from PEO spherulites is not completely perfect, and the entrapment of amorphous phase of PEO, PMA, and salt in PEO spherulites lead to suppression of PEO crystallinity as well as depression of apparent melting temperature of PEO in the systems, which will be discussed in the next section.
4.3. Melting Behavior
The apparent melting temperature (
Tm) was obtained from the maximum of the melting endotherm of the DSC curve.
Figure 4 depicts the apparent
Tm of PEO in the ternary mixtures as a function of the mole fraction of salt (
XS). As mentioned before, the decrease in the crystallinity of the crystalline phase is generally influenced by the miscibility of the mixture, which is also true for the apparent
Tm. The depression of the apparent
Tm of PEO is expected in miscible systems. We note here, both melting point and enthalpy are greatly depending on the amount of salt content. The apparent
Tms of PEO of the as-prepared samples are depressed significantly from the constancy curve with increasing salt content for all compositions. This implies that the dissolution of Li–salt in the polymer matrix (in PEO) increases, which is in good approximation to the
Tg as well as the crystallinity findings.
For binary polymer–salt mixtures with a crystalline constituent, we may employ the melting point depression of the crystalline polymer for estimation of deviations from perfect behavior [
25,
26]. In this case, when binary PEO/Li mixtures are completely miscible in the molten state, we employ the melting point depression of PEO for evaluation of the solid solution from perfect behavior (i.e., PEO–PEO
70 at
WS ≤ 0.10). The estimation is expressed by
where
XP denotes the mole fraction of PEO in the molten state, and
γP is the corresponding activity coefficient for the solution, whereas the quantities
Tmo and ∆
Href symbolize the melting temperature of neat PEO and the melting enthalpy of 100% crystallinity of PEO, respectively. For systems with low salt content (or the completely miscible systems, PEO–PEO
70 at
WS ≤ 0.10), we may assume ln
XP = −
XS. Hence, we see from Equation (8), the information about the activity coefficient
γP may be determined instantly from the linear plot of
Tm against
XS. This relationship is valid as long as the PEO crystalline phase crystallizes out from the molten mixture upon cooling from the melt. We apply Equation (8) to the as-prepared PEO/Li mixture for estimation of deviations from the ideal behavior (c.f. red-dashed curve in
Figure 4). From
Figure 4, we obtained ∆
T/K = 147.3
XS for the PEO/Li mixture. The deviation of melting point depression from perfect behavior [∆
T(
XS)] for the PEO/Li mixture, specifically at higher salt content (
XS > 0.05), is observed. This indicates at low salt contents that the melting point depression is not influenced by the salt, and it shows to a good approximation perfect behavior, displaying a very small
γP = 1 + 0.30
XS. This observation is also in good agreement with the findings discussed in references [
26,
27], where the estimation of the melting point depression of the high molar mass PEO (
Mη = 3 × 10
5 g mol
−1) against
XS under similar experimental conditions yields
γP close to unity (1 + 0.38
XS) as reported in reference [
27]. This indicates that the PEO/Li system under these experimental conditions behaves nearly perfectly. Similar trend observations are found for other miscible mixtures, such as PEO
90, PEO
80, and PEO
70, where drastic deviations only occur for systems with higher content of salt (
XS > 0.05). This implies that all systems that are still miscible with low salt contents under these experimental conditions behave nearly perfect as in binary PEO/Li systems. The results retrieved from Equation (8) are summarized in
Table 3.
4.4. Optical Microscopy
From
Tg measurements, we note the existence of single
Tgs for binary PEO/PMA blends, which is also true for ternary PEO/PMA/LiClO
4 mixtures at sufficiently small salt concentration under described experimental conditions. The heterogeneity (immiscibility) is observed only when the mass fraction of PEO is minor,
WPEO ≤ 0.6, and the mass fraction of salt is high,
WS ≥ 0.05. The optical micrographs of selected ternary PEO/PMA/LiClO
4 systems taken at 10× magnification is shown in
Table 4. The micrographs were captured at two different temperatures, i.e., 80 °C after 30 min (in the molten state) and 25 °C after 24 h (in the solid state). As expected, the binary PEO/PMA blends in the molten state (
T = 80 °C) display only a single-phase without any visible boundary with polymer interfaces. This observation is also true for ternary mixtures with small concentration of salt (i.e., systems of PEO, PEO
80, and PEO
50 without the presence of salt). The two-phase structure occurs when the mass fraction of PEO is minor,
WPEO ≤ 0.6, and mass fraction salt is high,
WS ≥ 0.05. It is supported by the micrographs of PEO
50 after addition of
WS ≥ 0.05, where a two-phase structure with clear boundaries at the polymer interface is observed. PEO
50 with
WS = 0.065 displays a matrix-droplet morphology, and it starts to transform into co-continuous morphology with addition of higher salt content at
WS = 0.091. This observation on PEO
50 with salt may be associated to the situation (I) and (IV) as described in the introduction, where the liquid–liquid phase separation is inferred in the melt and amorphous phase of the semi-crystalline/amorphous PEO/PMA blends after addition of higher content of salt (this is in good agreement to the
Tg results; system with
WPEO ≤ 0.6 at
WS ≥ 0.05, which possess two
Tgs that correspond to PEO and PMA).
At 25 °C, where PEO is crystalline below the melting point Tm = ~65 °C, the samples are placed between crossed polarizers of the optical microscope. Using polarized light, the neat PEO displays large spherulites with a fine fibrillar texture and a clear Maltese cross. As expected, the number of nucleation sites of PEO spherulites for PEO, PEO80, and PEO50 increase with and without salt due to the exclusion of PMA and Li–salt from the PEO crystalline phase during crystallization This is also reflected by the Tm results, where the melting point depression of PEO is observed with increasing salt content. This is due the fact that PMA and Li–salt most likely have attractive interactions with PEO phases, which eventually lead to the incomplete exclusion. Coarsening and irregularity of PEO spherulite fibrils with blurred grain boundary are clearly noted for all systems at high salt content. There are several spherical dark spots of PMA within the continuous phase of PEO spherulites for PEO50 at WS = 0.065 and 0.091.
The proposed schematic diagrams of PEO
50 systems at
WS = 0 and 0.091 in the molten and solid state based on the
Tg results and polarized optical microscopy are shown in
Figure 5. We conclude that both systems possess different morphologies molten at 80 °C or isothermally crystallized at 25 °C. Immiscibility caused by liquid–liquid phase separation (at 80 °C) (c.f.
Figure 5c) and liquid–solid phase separation in PEO
50 are observed while cooling down from 80 °C to 25 °C (c.f.
Figure 5d) after addition of salt
WS = 0.091.
Figure 5 was assigned with the possible morphologies from
Table 1.
4.5. Impedance Spectroscopy
The impedance spectra of all systems were measured at 25 °C. Selected binary systems of PEO, PMA, and its blends, such as PEO
90, PEO
80, PEO
70, PEO
60 and PEO
50, added with different salt content will be discussed under this section, where the morphologies of these systems are correlated to situation (I), (II), (III), or (IV). It is widely accepted that the percolation network only bounds in the amorphous domain of salt-added systems. This phase is considered as not in thermodynamic equilibrium [
28,
29,
30,
31]. It may be adequate to discuss the dielectric relaxation process in this domain as a fluctuation–dissipation process [
32]. Hence, in the following, the development of relaxation or polarization of dipoles are discussed from the phenomenological point of view.
We observe here that the real part (
Z’) and imaginary part (
Z″) of impedance reflect to Ohmic resistance and non-Ohmic resistance, respectively. The quantity
Z″ displays the characteristic frequencies mainly for dielectric (or dipole) relaxation resulting from local motions of the charged entities and electrode polarization developing from the accumulation of charged entities at the electrode–electrolyte interfaces, after being subjected to an external electric field. These characteristic frequencies are noted as
and
, respectively. Besides, the intersection between
Z’ and
Z″ that is noted as
indicates to the development of the percolation charge entities. We recognize the characteristic frequencies as the average of relaxation time constant (
ω−1). A system with one relaxation time (
ω−1) is recognized as Debye relaxation [
33]. In general, these phenomena can be observed in a system with highly resistive behavior. A system with high capacitive behavior normally behaves contrarily. Apparently, the impedance plot
Z″ =
Z″ (
Z’) in the
Z’-
Z″ plane results in semicircle with a radius of
Rb/2. Hence, the plot of
Z″ vs. frequency (
f) as displayed in
Figure 6 allows for the determination of the bulk resistance (
Rb), with |
Z″max| =
Rb/2. This
Rb quantity is defined as inversely proportional to the DC conductivity (
Rb ∝ 1/
σDC). Hence, investigation of the relaxation process in terms of impedance from phenomenological perspective may implicitly elucidate the dielectric and electrical behavior of the system.
Figure 6 represents the impedance spectra,
Z’ (
f) and
Z″ (
f), of PEO at low salt content
WS = 0.0196. We observe the existence of the three characteristic frequencies as mentioned before in the impedance spectra of the system. The
is noted as the main dipole relaxation, which originates from the alignment of dipoles resulting from the interplay of the sample’s resistance and capacitance in the fully stabilized network. The
is noted as the electrode polarization (or called a double-layer capacitance due to the accumulation of charges at the interface of electrode and electrolyte) especially at low frequency. This observation frequently leads to dispersion of relaxation times (i.e.,
<
) and normally becomes severe in the system with high salt content because of the inhomogeneity in the system (c.f. red triangle markers for PEO–salt in
Figure 7). However, the distance of
and
for PEO at
WS = 0.0196 is small which may indicate that the system is close to Debye relaxation. We suggest that the orientation of dipolar entities in PEO–salt is dominantly restricted to short-range motion (local motion) and insignificant long-range motion. The PMA–salt has no
at low salt content and one broad
is observed in
Z″ spectrum of system with high salt content at frequency. This indicates no dipolar entities in PMA with low salt content and only appears after adding higher content of salt under the experimental condition.
Figure 7 displays the impedance spectra of PEO/PMA mixtures at different
WS. PEO, PMA, and its blends with salt are above the glassy state of parent polymers (c.f. glass transition temperature).
Rb values clearly decrease with increasing salt content for all systems and this indirectly infers the increasing DC conductivity. For system with PEO in excess, under
WS =
const., either for low or high content of salt, the
Rb decreases slightly as compared to the binary PEO/Li salt system. This may indicate that the system with PEO in excess behaves similar to PEO especially at low salt content, for instance, PEO
80 at
WS = 0.0196 (low content of salt). A large distribution of relaxation times is observed for PEO and PEO
80 with high salt content
WS = 0.0909 as well as for PEO
50 at
WS = 0.0196–0.0909 and PMA at
WS = 0.0909, based on the observation of the characteristic frequencies
<
at the respective compositions. Additionally, the presence of
, which points towards electrode polarization, may also contributes to the distribution of relaxation times of a system (i.e., PEO–PEO
50 at
WS ≥ 0.0196 in
Figure 7). This deviation may indicate to non-Debye response that normally occurs because of the increase in inhomogeneity in the system. We may summarize that, at high salt content, the distribution of characteristic frequencies is always denoted as
whereas at low salt content, it is seen as
At WS = const. especially at high salt content, when PMA is in excess or in symmetric systems (i.e., WPEO ≤ 0.5) Rb and characteristic frequencies shift to higher and lower values, respectively, as compared to binary PEO/Li salt blends or system with PEO in excess. It is noted that these systems are seen as liquid–liquid phase separated as reflected in the Tg results of the same system, where situations (I) and (IV) are correlated. This behavior may indicate preferential localization of salt in the different polymer phases of the immiscible ternary mixture. For instance, based on the Tg results, we may assume that the PEO phase in PEO50 at WS = 0.091 contains less salt as compared to the binary PEO/Li salt system at the same composition. This phenomenon might be one of the reasons for the enhancement of the bulk resistance as well as large deviation from Debye response in the liquid–liquid phase separated systems.
Further insight,
Figure 7 illustrates that impedance spectra of PEO/PMA blends comprise low and high concentration of salt, respectively. Interesting points of the impedance spectra are suggested as follows. Blends with PEO in excess show similar behavior of neat PEO at low salt concentration. The amorphous network-like phase of PEO in the blends is almost not influenced by the PMA phase. The PEO amorphous phase contains slightly more salt than in the neat PEO; as a consequence, bulk resistance is slightly reduced. Additionally, we note that
and
. Enhanced content of PMA in blends leads increasingly to higher
Rb at
YS =
const. On top of that,
shift to lower values. It indicates “effective″ salt content of the amorphous PEO phase lessens more and more. Again, one observes more dispersion of relaxation times,
. In short, dielectric behavior of PEO and of the blends with PMA is governed by amorphous network-like phases. Addition of Li–salt leads to dissolution of low “effective″ amounts of salt in these phases. Therefore, corresponding polymer–salt mixture can be seen to good approximation as a perfect solution. This PEO-like behavior is maintained when low content PMA is added to PEO at room temperature.
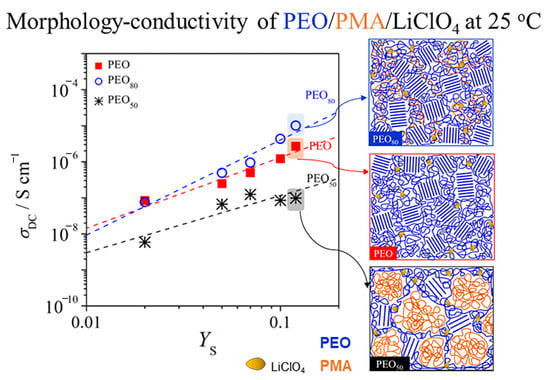
Figure 8 depicts the double-logarithmic plots of conductivity (
σDC) as a function of salt concentration (
YS). The salt concentration
YS is defined as the ratio of mass of salt to the mass of polymer. The double-logarithmic plots of
σDC vs.
YS for PEO/PMA/LiClO
4 systems denote a power law dependence resulting from the functional relationship between the two quantities [
27,
34]. The linear relationship of the double-logarithmic plot of
σDC vs.
YS can be read as
which yields the quantity of
σo and allows for the estimation of the exponent
x [
27,
34]. The exponent
x in Equation (11) is the extent of association between salt molecules and the polymer segments. The quantity
σo in Equation (11) is the simplified form of charge mobility in the system. The full form equation can be read as
where
μα is the mobility,
NA is the Avogadro’s constant,
e is the elementary charge,
ρp is the polymer’s density, and
MS represents the molar mass of LiClO
4, where the molecular characteristics for determination of quantity
μα are listed in
Table 1. The density of each blend was calculated using the additivity rule. All characteristic quantities of the linear regression function for all systems adopted from Equation (12) are summarized in
Table 5.
From
Figure 8, binary PMA/Li salt systems display the lowest
σDC as compared to the binary PEO/Li salt blends and in the blends of PEO/PMA systems due to the fact that the
μα mobility in PMA is by three orders of magnitude lower than in PEO. Moreover, PEO has stronger association between salt molecules and polymer segments than salt in PMA, as reflected in exponent
x = 1.96 for PEO as compared to
x = 1.59 for PMA, which might be the reason for lower
σDC of PMA. This indicates that the salts interact with PMA very weakly, which is in agreement with the
Tg findings of the systems. PEO
80 at
WS = 0.107 has the highest value of ion conductivity at 1.43 × 10
−5 S cm
−1 as compared to the other blend compositions with
WS =
const. This is in good agreement where PEO
80 also has the highest value of exponent
x = 2.28 and
μα = 3.81 × 10
−6 cm
2 V
−1 s
−1 as compared to the other blend compositions. The blends
WPEO = 0.5 exhibit lower values of
μα mobility than PEO but higher than
μα mobility of PMA. Hence, we may conclude the ionic conductivities of PEO/PMA/Li blends are dominated by the percolation of PEO in the amorphous phase. Blending small contents of PMA with PEO, when PEO in excess with addition of salt enhances the
σDC of systems as compared to binary systems of PEO and salt with
WS =
const. However, the conductivities of the symmetric system, i.e., PEO
50, decrease by two orders of magnitude as compared to PEO–salt systems. This might be caused by the fact that the Li cation is not coordinating well with the chains of both polymers for the phase-separated systems at room temperature. Therefore, we may assume that when PEO with addition of salt is dispersed in PMA domains such as for systems
WPEO ≤ 0.5 where the Li–salt in the PEO phase may not have sufficient percolating paths unlike the systems with PEO in excess.