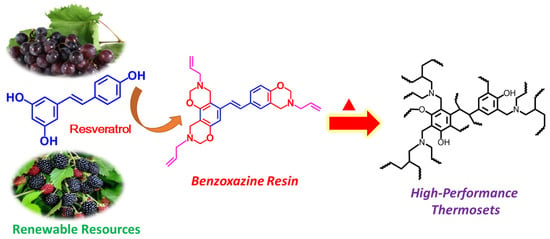
Design of High-Performance Polybenzoxazines with Tunable Extended Networks Based on Resveratrol and Allyl Functional Benzoxazine
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Synthesis of Tri-Functional Benzoxazine (RES-al)
2.4. Polymerization Procedures of RES-al
3. Results and Discussion
3.1. Synthesis and Structural Confirmation of RES-al
3.2. Polymerization Behaviors of the Benzoxazine Monomer
3.3. Thermal Properties of Thermosets Derived from RES-al
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ning, X.; Ishida, H. Phenolic materials via ring-opening polymerization: Synthesis and characterization of bisphenol-A based benzoxazines and their polymers. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1121–1129. [Google Scholar] [CrossRef]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Ishida, H.; Froimowicz, P. Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ishida, H.; Agag, T. Handbook of Benzoxazine Resins; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Shen, X.; Cao, L.; Liu, Y.; Dai, J.; Liu, X.; Zhu, J.; Du, S. How does the hydrogen bonding interaction influence the properties of polybenzoxazine? An experimental study combined with computer simulation. Macromolecules 2018, 51, 4782–4799. [Google Scholar] [CrossRef]
- Ohashi, S.; Kilbane, J.; Heyl, T.; Ishida, H. Synthesis and characterization of cyanate ester functional benzoxazine and its polymer. Macromolecules 2015, 48, 8412–8417. [Google Scholar] [CrossRef]
- Liu, J.; Safronava, N.; Lyon, R.E.; Maia, J.; Ishida, H. Enhanced thermal property and flame retardancy via intramolecular 5-membered ring hydrogen bond-forming amide functional benzoxazine resins. Macromolecules 2018, 51, 9982–9991. [Google Scholar] [CrossRef]
- Kolanadiyil, S.N.; Bijwe, J.; Varma, I.K. Synthesis of itaconimide/nadimide-functionalized benzoxazine monomers: Structural and thermal characterization. React. Funct. Polym. 2013, 73, 1544–1552. [Google Scholar] [CrossRef]
- Yang, P.; Gu, Y. Synthesis of a novel benzoxazine-containing benzoxazole structure and its high performance thermoset. J. Appl. Polym. Sci. 2012, 124, 2415–2422. [Google Scholar] [CrossRef]
- Chaisuwan, T.; Ishida, H. High-performance maleimide and nitrile-functionalized benzoxazines with good processibility for advanced composites applications. J. Appl. Polym. Sci. 2006, 101, 548–558. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, X. Catalyst-free and low-temperature terpolymerization in a single-component benzoxazine resin containing both norbornene and acetylene functionalities. Macromolecules 2018, 51, 6524–6533. [Google Scholar] [CrossRef]
- Chen, S.; Ren, D.; Li, B.; Li, K.; Chen, L.; Xu, M.; Liu, X. Benzoxazine containing fluorinated aromatic ether nitrile linkage: Preparation, curing kinetics and dielectric properties. Polymers 2019, 11, 1036. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Xi, Y.; Mccandless, G.T.; Xie, Y.; Menon, R.; Patel, Y.; Menon, R.; Patel, Y.; Yang, D.J.; Iacono, S.T.; et al. Synthesis and characterization of partially fluorinated polybenzoxazine resins utilizing octafluorocyclopentene as a versatile building block. Macromolecules 2015, 48, 6087–6095. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, X.; Kuo, S.W. Outstanding dielectric and thermal properties of main chain-type poly(benzoxazine-co-imide-co-siloxane)-based cross-linked networks. Polym. Chem. 2019, 10, 2387–2396. [Google Scholar] [CrossRef]
- Sini, N.K.; Endo, T. Toward elucidating the role of number of oxazine rings and intermediates in the benzoxazine backbone on their thermal characteristics. Macromolecules 2016, 49, 8466–8478. [Google Scholar] [CrossRef]
- Kolanadiyil, S.N.; Azechi, M.; Endo, T. Synthesis of novel tri-benzoxazine and effect of phenolic nucleophiles on its ring-opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2811–2819. [Google Scholar] [CrossRef]
- Zhang, K.; Han, M.C.; Liu, Y.; Froimowicz, P. Design and synthesis of bio-based high performance tri-oxazine benzoxazine resin via natural renewable resources. ACS Sustain. Chem. Eng. 2019, 7, 9399–9407. [Google Scholar] [CrossRef]
- Zhang, K.; Han, M.C.; Han, L.; Ishida, H. Resveratrol-based tri-functional benzoxazines: Synthesis, characterization, polymerization, and thermal and flame retardant properties. Eur. Polym. J. 2019, 116, 526–533. [Google Scholar] [CrossRef]
- Wang, D.; Li, B.; Zhang, Y.; Lu, Z. Triazine-containing benzoxazine and its high-performance polymer. J. Appl. Polym. Sci. 2013, 127, 516–522. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Alhassan, S.M.; Katsiotis, M.S.; Ishida, H.; Qutubuddin, S. Interactions, Morphology and Thermal Stability of Graphene-Oxide Reinforced Polymer Aerogel Derived from Star-Like Telechelic Aldehyde- Terminal Benzoxazine Resin. RSC Adv. 2015, 5, 92719–92731. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Poly(benzoxazine-f-chitosan) films: The role of aldehyde neighboring groups on chemical interaction of benzoxazine precursors with chitosan. Carbohyd. Polym. 2019, 209, 122–129. [Google Scholar] [CrossRef]
- Cash, J.J.; Davis, M.C.; Ford, M.D.; Groshens, T.J.; Guenthner, A.J.; Harvey, B.G.; Lamison, K.R.; Mabry, J.M.; Meylemans, H.A.; Reams, J.T.; et al. High Tg thermosetting resins from resveratrol. Polym. Chem. 2013, 4, 3859–3865. [Google Scholar] [CrossRef]
- Vervandier-Fasseur, D.; Chalal, M.; Meunier, P. Method for Producing Trans-Resveratrol and the Analogs Thereof. WO Patent No. 2013008175A, 17 January 2013. [Google Scholar]
- Subbaraju, G.V.; Mahesh, M.; Hindupur, H.R.; Suresh, T.; Ivanisevic, I.; Andres, M.; Stephens, K. Key Intermediate for the Preparation of Stilbenes, Solid Forms of Pterostilbene, and Methods for Making the Same. U.S. Patent 20110144212 A1, 16 June 2011. [Google Scholar]
- Fan, E.; Zhang, K.; Zhu, M.; Wang, Q. Obtaining resveratrol: From chemical synthesis to biotechnology production. Mini-Rev. Org. Chem. 2010, 7, 272–281. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Q.; Shen, L.; Cui, Z.; Kou, L.; Cheng, J.; Zhang, J. A renewable resveratrol-based epoxy resin with high Tg, excellent mechanical properties and low flammability. Chem. Eng. J. 2020, 383, 12124. [Google Scholar] [CrossRef]
- Sawaryn, C.; Landfester, K.; Taden, A. Benzoxazine miniemulsions stabilized with polymerizable nonionic benzoxazine surfactants. Macromolecules 2010, 43, 8933–8941. [Google Scholar] [CrossRef]
- Dunkers, J.; Ishida, H. Vibrational assignments of 3-alkyl-3, 4-dihydro-6-methyl-2H-1,3-benzoxazines in the Fingerprint Region. Spectrochim. Acta Part A 1995, 51, 1061–1074. [Google Scholar] [CrossRef]
- Han, L.; Iguchi, D.; Gil, P.; Heyl, T.R.; Sedwick, V.M.; Arza, C.R.; Ohashi, S.; Lacks, D.J.; Ishida, H. Oxazine ring-related vibrational modes of benzoxazine monomers using fully aromatically substituted, deuterated, 15N isotope exchanged, and oxazine-ring-substituted compounds and theoretical calculations. J. Phys. Chem. A 2017, 121, 6269–6282. [Google Scholar] [CrossRef]
- Sini, N.K.; Azechi, M.; Endo, T. Synthesis and properties of spiro-centered benzoxazines. Macromolecules 2015, 48, 7466–7472. [Google Scholar]
- Zhang, K.; Shang, Z.; Evans, C.J.; Han, L.; Ishida, H.; Yang, S. Benzoxazine atropisomers: Intrinsic atropisomerization mechanism and conversion to high performance thermosets. Macromolecules 2018, 51, 7574–7585. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Evans, C.J.; Yang, S.F. Easily processable thermosets with outstanding performance via smart twisted small-molecule benzoxazines. Macromol. Rapid Commun. 2020, 41, 1900625. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.; Liu, W.; Yang, S.; Bai, J.; Ding, Q.; Li, Y. Synthesis, curing behavior and thermal properties of fluorine containing benzoxazines. Eur. Polym. J. 2010, 46, 1024–1031. [Google Scholar] [CrossRef]
- Zhang, K.; Tan, X.X.; Wang, Y.T.; Ishida, H. Unique self-catalyzed cationic ring-opening polymerization of a high performance deoxybenzoin-based 1,3-benzoxazine monomer. Polymer 2019, 168, 8–15. [Google Scholar] [CrossRef]
- Hamerton, I.; Thompson, S.; Howlin, B.J.; Stone, C.A. New method to predict the thermal degradation behavior of polybenzoxazines from empirical data using structure property relationships. Macromolecules 2013, 46, 7605–7615. [Google Scholar] [CrossRef]











| Sample | Tg (DMA) | N2 | Air | |||
|---|---|---|---|---|---|---|
| (°C) | Td5 (°C) | Td10 (°C) | Yc | Td5 (°C) | Td10 (°C) | |
| poly(RES-al)-1 | 230 | 318 | 342 | 45 | 290 | 328 |
| poly(RES-al)-2 | 274 | 338 | 363 | 48 | 321 | 349 |
| poly(RES-al)-3 | 313 | 352 | 378 | 53 | 352 | 380 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; He, X.; Yang, R.; Zhang, K.; Yang, S. Design of High-Performance Polybenzoxazines with Tunable Extended Networks Based on Resveratrol and Allyl Functional Benzoxazine. Polymers 2020, 12, 2794. https://doi.org/10.3390/polym12122794
Xing Y, He X, Yang R, Zhang K, Yang S. Design of High-Performance Polybenzoxazines with Tunable Extended Networks Based on Resveratrol and Allyl Functional Benzoxazine. Polymers. 2020; 12(12):2794. https://doi.org/10.3390/polym12122794
Chicago/Turabian StyleXing, Yunliang, Xianru He, Rui Yang, Kan Zhang, and Shengfu Yang. 2020. "Design of High-Performance Polybenzoxazines with Tunable Extended Networks Based on Resveratrol and Allyl Functional Benzoxazine" Polymers 12, no. 12: 2794. https://doi.org/10.3390/polym12122794