UCST Type Phase Boundary and Accelerated Crystallization in PTT/PET Blends
Abstract
:1. Introduction
2. Materials and Methods
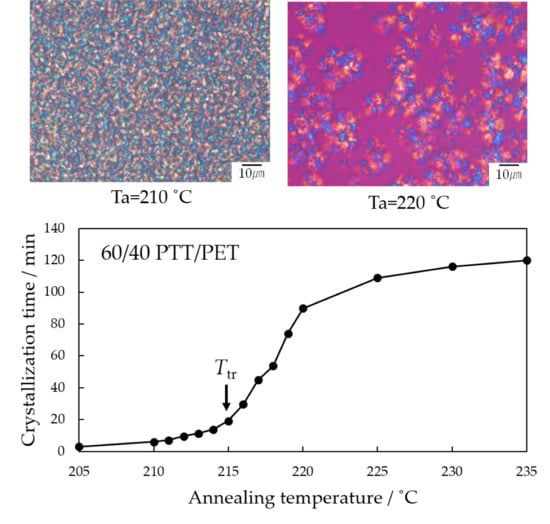
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olabis, O.; Robeson, L.M.; Shaw, M.T. Polymer-Polymer Miscibility, 1st ed.; Elsevier: New York, NY, USA, 1979. [Google Scholar]
- Paul, D.R.; Newman, S. Polymer Blends; Elsevier: New York, NY, USA, 1978; Volume 1. [Google Scholar]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook, 2nd ed.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Thomas, S.; Grohens, Y.; Jyotishkumar, P. Characterization of Polymer Blends: Miscibility, Morphology and Interfaces; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Ougizawa, T.; Inoue, T.; Kammer, H.W. UCST and LCST behavior in polymer blends. Macromolecules 1985, 18, 2089–2092. [Google Scholar] [CrossRef]
- Ougizawa, T.; Inoue, T. UCST and LCST behavior in polymer blends and its thermodynamic interpretation. Polym. J. 1986, 18, 521–527. [Google Scholar] [CrossRef]
- Kawahara, S.; Akiyama, S. UCST phase behavior and the miscibility valley in blends of poly (vinyl ethylene-co-1, 4-butadiene) and hydrogenated terpene resin. Macromolecules 1993, 26, 2428–2432. [Google Scholar] [CrossRef]
- Sato, T.; Endo, M.; Shiomi, T.; Imai, K. UCST behaviour for high-molecular-weight polymer blends and estimation of segmental χ parameters from their miscibility. Polymer 1996, 37, 2131–2136. [Google Scholar] [CrossRef]
- Yang, Q.; Mao, Y.; Li, G.; Huang, Y.; Tang, P.; Lei, C. Study on the UCST behavior of polystyrene/poly (styrene-co-acrylonitrile) blend. Mater. Lett. 2004, 58, 3939–3944. [Google Scholar] [CrossRef]
- Saito, H.; Fujita, Y.; Inoue, T. Upper Critical Solution Temperature Behavior in Poly(vinylidene fluoride)/Poly(methyl methacrylate) Blends. Polym. J. 1987, 19, 405–412. [Google Scholar] [CrossRef]
- Tomura, H.; Saito, H.; Inoue, T. Light Scattering Analysis of Upper Critical Solution Temperature Behavio in a Poly(vinylidene fluoride/Poly(methyl methacrylate) Blend. Macromolecules 1992, 25, 1611–1614. [Google Scholar] [CrossRef]
- Lee, C.; Saito, H.; Goizueta, G.; Inoue, T. An immiscibility loop in isotactic polypropylene/partially hydrogenated oligo(styrene-co-indene) blend. Macromolecules 1996, 29, 4274–4277. [Google Scholar] [CrossRef]
- Chen, H.-L.; Hwang, J.C.; Chen, C.-C.; Wang, R.-C.; Fang, D.-M.; Tsai, M.-J. Phase behaviour of amorphous and semicrystalline blends of poly (butylene terephthalate) and poly (ether imide). Polymer 1997, 38, 2747–2752. [Google Scholar] [CrossRef]
- Chen, H.-L.; Hwang, J.C.; Yang, J.-M.; Wang, R.-C. Simultaneous liquid–liquid demixing and crystallization and its effect on the spherulite growth in poly (ethylene terephthalate)/poly (ether imide) blends. Polymer 1998, 39, 6983–6989. [Google Scholar] [CrossRef]
- Tsuburaya, M.; Saito, H. Crystallization of polycarbonate induced by spinodal decomposition in polymer blends. Polymer 2004, 45, 1027–1032. [Google Scholar] [CrossRef]
- Woo, E.M.; Cheng, K.Y.; Chen, Y.-F.; Su, C. Experimental verification on UCST phase diagrams and miscibility in binary blends of isotactic, syndiotactic, and atactic polypropylenes. Polymer 2007, 48, 5753–5766. [Google Scholar] [CrossRef]
- Nurkhamidah, S.; Woo, E.M. Phase Separation and Lamellae Assembly below UCST in Poly (l-lactic acid)/Poly (1, 4-butylene adipate) Blend Induced by Crystallization. Macromolecules 2012, 45, 3094–3103. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Muthukumar, M.; Han, C.C. Fluctuation-assisted crystallization: In a simultaneous phase separation and crystallization polyolefin blend system. Macromol. Rapid Commun. 2005, 26, 1285–1288. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Dong, X.; Wang, D.; Han, C.C. Interplay between two phase transitions: Crystallization and liquid-liquid phase separation in a polyolefin blend. J. Chem. Phys. 2006, 125, 024907. [Google Scholar] [CrossRef]
- Ong, C.; Price, F. Blends of poly (ϵ-Caprolactone) with poly (vinyl chloride). II. Crystallization kinetics. J. Polym. Sci. Part C Polym. Symp. 1978, 63, 59–75. [Google Scholar]
- Alfonso, G.; Russell, T. Kinetics of crystallization in semicrystalline/amorphous polymer mixtures. Macromolecules 1986, 19, 1143–1152. [Google Scholar] [CrossRef]
- Briber, R.M.; Khoury, F. The phase diagram and morphology of blends of poly (vinylidene fluoride) and poly (ethyl acrylate). Polymer 1987, 28, 38–46. [Google Scholar] [CrossRef]
- Chow, T. Miscible blends and block copolymers. Crystallization, melting, and interaction. Macromolecules 1990, 23, 333–337. [Google Scholar] [CrossRef]
- Saito, H.; Okada, T.; Hamane, T.; Inoue, T. Crystallization kinetics in Mixtures of poly(vinylidene fluoride) and poly(methyl methacrylate): Two-step diffusion mechanism. Macromolecules 1991, 24, 4446–4449. [Google Scholar] [CrossRef]
- Penning, J.; St. John Manley, R. Miscible blends of two crystalline polymers. 2. Crystallization kinetics and morphology in blends of poly (vinylidene fluoride) and poly (1, 4-butylene adipate). Macromolecules 1996, 29, 84–90. [Google Scholar] [CrossRef]
- Chen, H.-L.; Li, L.-J.; Lin, T.-L. Formation of segregation morphology in crystalline/amorphous polymer blends: Molecular weight effect. Macromolecules 1998, 31, 2255–2264. [Google Scholar] [CrossRef]
- Zhang, L.; Goh, S.; Lee, S.; Hee, G. Miscibility, melting and crystallization behavior of two bacterial polyester/poly (epichlorohydrin-co-ethylene oxide) blend systems. Polymer 2000, 41, 1429–1439. [Google Scholar] [CrossRef]
- Okabe, Y.; Murakami, H.; Osaka, N.; Saito, H.; Inoue, T. Morphology development and exclusion of noncrystalline polymer during crystallization in PVDF/PMMA blends. Polymer 2010, 51, 1494–1500. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N.; Panayiotou, C.G. Novel miscible poly(ethylene sebacate)/poly(4-vinyl phenol) blends: Miscibility, melting behavior and crystallization study. Polymer 2011, 52, 4553–4561. [Google Scholar] [CrossRef]
- Ma, Y.; Zha, L.; Hu, W.; Reiter, G.; Han, C.C. Crystal nucleation enhanced at the diffuse interface of immiscible polymer blends. Phys. Rev. E 2008, 77, 061801. [Google Scholar] [CrossRef]
- Hu, W. Interplay of Liquid-Liquid Demixing and Polymer Crystallization. In Understanding Soft Condensed Matter via Modeling and Computation; Hu, W., Shi, A.-C., Eds.; World Scientific: Singapore, 2011; Volume 3. [Google Scholar]
- Hu, W. Polymer Physics: A Molecular Approach; Springer-Verlag: Wien, Austria, 2013. [Google Scholar]
- Hong, P.-D.; Chung, W.-T.; Hsu, C.-F. Crystallization kinetics and morphology of poly (trimethylene terephthalate). Polymer 2002, 43, 3335–3343. [Google Scholar] [CrossRef]
- Chuang, W.-T.; Hong, P.-D.; Chuah, H.H. Effects of crystallization behavior on morphological change in poly (trimethylene terephthalate) spherulites. Polymer 2004, 45, 2413–2425. [Google Scholar] [CrossRef]
- Supaphol, P.; Dangseeyun, N.; Thanomkiat, P.; Nithitanakul, M. Thermal, crystallization, mechanical, and rheological characteristics of poly (trimethylene terephthalate)/poly (ethylene terephthalate) blends. J. Polym. Sci. Part B: Polym. Phys. 2004, 42, 676–686. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Woo, E.M. Miscibility in two blend systems of homologous semicrystalline aryl polyesters involving poly (trimethylene terephthalate). Polym. J. 2003, 35, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Castellano, M.; Turturro, A.; Valenti, B.; Avagliano, A.; Costa, G. Reactive Blending of Aromatic Polyesters: Thermal Behaviour of Co-precipitated Mixtures PTT/PET. Macromol. Chem. Phys. 2006, 207, 242–251. [Google Scholar] [CrossRef]
- Run, M.; Hao, Y.; Yao, C. Melt-crystallization behavior and isothermal crystallization kinetics of crystalline/crystalline blends of poly (ethylene terephthalate)/poly (trimethylene terephthalate). Thermochim. Acta 2009, 495, 51–56. [Google Scholar] [CrossRef]
- Kim, G.S.; Son, J.M.; Lee, J.K.; Lee, K.H. Morphology development and crystallization behavior of poly (ethylene terephthalate)/poly (trimethylene terephthalate) blends. Eur. Polym. J. 2010, 46, 1696–1704. [Google Scholar] [CrossRef]
- Stein, R.S.; Rhodes, M.B. Photographic light scattering by polyethylene films. J. Appl. Phys. 1960, 31, 1873–1884. [Google Scholar] [CrossRef]
- Lee, C.H.; Saito, H.; Inoue, T. Time-resolved light scattering studies on the early stage of crystallization in poly(ethylene terephthalate). Macromolecules 1993, 26, 6566–6569. [Google Scholar] [CrossRef]
- Koberstein, J.; Russell, T.; Stein, R. Total integrated light-scattering intensity from polymeric solids. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 1719–1730. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugeno, K.; Kokubun, S.; Saito, H. UCST Type Phase Boundary and Accelerated Crystallization in PTT/PET Blends. Polymers 2020, 12, 2730. https://doi.org/10.3390/polym12112730
Sugeno K, Kokubun S, Saito H. UCST Type Phase Boundary and Accelerated Crystallization in PTT/PET Blends. Polymers. 2020; 12(11):2730. https://doi.org/10.3390/polym12112730
Chicago/Turabian StyleSugeno, Kousuke, Satoshi Kokubun, and Hiromu Saito. 2020. "UCST Type Phase Boundary and Accelerated Crystallization in PTT/PET Blends" Polymers 12, no. 11: 2730. https://doi.org/10.3390/polym12112730