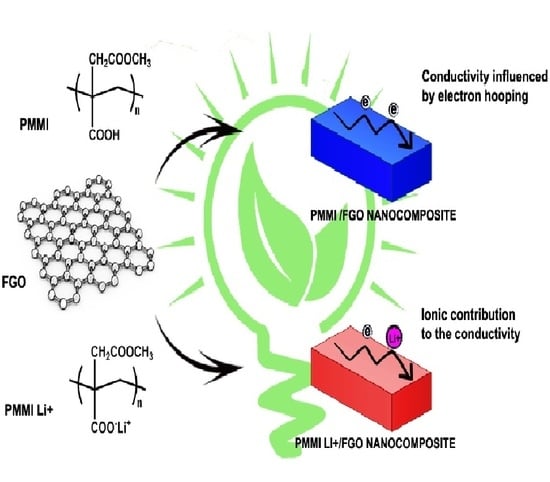
Electrical Properties of Poly(Monomethyl Itaconate)/Few-Layer Functionalized Graphene Oxide/Lithium Ion Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(Monomethyl Itaconate) and Poly(Monomethyl Itaconate) Li+
2.3. Preparation of Nanocomposites
2.4. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmaiah, M.; Afrin, S.; Malladi, R.P.; Elkoun, S.; Robert, M.; Ansari, M.A.; Svedberg, A.; Karim, Z. Chapter 9—Biocomposites: Present trends and challenges for the future. In Woodhead Publishing Series in Composites Science and Engineering; Koronis, G., Silva, Eds.; Woodhead Publishing: Duxford, UK, 2019; ISBN 978-0-08-102177-4. [Google Scholar]
- Karaffa, L.; Kubicek, C.P. Production of organic acids by fungi. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-809633-8. [Google Scholar]
- Schildknecht, C.E. Vinyl and Related Polymers; John Wiley and Sons: New York, NY, USA, 1952; 723p. [Google Scholar] [CrossRef]
- Marvel, C.S.; Shepherd, T.H. Polymerization reactions of itaconic acid and some of its derivatives. J. Org. Chem. 1959, 24, 599–605. [Google Scholar] [CrossRef]
- Cowie, J.M.G. Physical properties of polymers based on itaconic acid. Pure Appl. Chem. 1979, 51, 2331–2343. [Google Scholar] [CrossRef]
- Pérocheau Arnaud, S.; Andreou, E.; Pereira Köster, L.V.; Robert, T. Selective synthesis of monoesters of itaconic acid with broad substrate scope: Biobased alternatives to acrylic Acid? ACS Sustain. Chem. Eng. 2019, 8, 1583–1590. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Yazdani Pedram, M.; Ferguson, R. Poly(alkyl itaconate)s. formation of mixed esters and the properties of the resulting polymers. Eur. Polym. J. 1985, 21, 227–232. [Google Scholar] [CrossRef]
- Bonardd, S.; Alegria, A.; Saldias, C.; Leiva, A.; Kortaberria, G. Polyitaconates: A new family of “all-polymer” dielectrics. ACS Appl. Mater. Interfaces 2018, 10, 38476–38492. [Google Scholar] [CrossRef]
- Nasreen, S.; Treich, G.M.; Baczkowski, M.L.; Mannodi-Kanakkithodi, A.K.; Cao, Y.; Ramprasad, R.; Sotzing, G. Polymer dielectrics for capacitor application. In Kirk-Othmer Encyclopedia of Chemical Technology; Othmer, D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–29. ISBN 9780471484943. [Google Scholar]
- Cesteros, L.C.; Velada, J.; Katime, I. Polymer—polymer complexation between poly(monomethyl itaconate) and poly(vinylpyridine)s. Polymer 1995, 36, 3183–3189. [Google Scholar] [CrossRef]
- Riande, E.; Díaz-Calleja, R. Electrical properties of polymers. In Handbook of Measurement of Science and Engineering; Kutz, M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 1291–1342. ISBN 9781118436707. [Google Scholar]
- Guan, L.-Z.; Zhao, L.; Wan, Y.-J.; Tang, L.-C. Three-dimensional graphene-based polymer nanocomposites: Preparation, properties and applications. Nanoscale 2018, 10, 14788–14811. [Google Scholar] [CrossRef]
- Zhen, Z.; Zhu, H. Structure and properties of graphene. In Graphene: Fabrication, Characterizations, Properties and Applications; Zhu, H., Xu, Z., Xie, D., Fang, Y., Eds.; Academic Press: London, UK, 2018; ISBN 978-0-12-812651-6. [Google Scholar]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family – A recommended nomenclature for two-dimensional carbon materials. Carbon N. Y. 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Ji, X.; Xu, Y.; Zhang, W.; Cui, L.; Liu, J. Review of functionalization, structure and properties of graphene/polymer composite fibers. Compos. Part. A Appl. Sci. Manuf. 2016, 87, 29–45. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Verdejo, R. Thermal, electrical, and sensing properties of rubber nanocomposites. In High-Performance Elastomeric Materials Reinforced by Nano-Carbons; Valentini, L., Lopez-Manchado, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–175. [Google Scholar] [CrossRef]
- Romasanta, L.J.; Lopez-Manchado, M.A.; Verdejo, R. Increasing the performance of dielectric elastomer actuators: A review from the materials perspective. Prog. Polym. Sci. 2015, 51, 188–211. [Google Scholar] [CrossRef] [Green Version]
- Dhatarwal, P.; Sengwa, R.J. Dielectric polarization and relaxation processes of the lithium-ion conducting PEO/PVDF blend matrix-based electrolytes: Effect of TiO2 nanofiller. SN Appl. Sci. 2020, 2, 833. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Gallegos-Cofre, M. Highly Electrical Conductive Composite Material Based on Graphene-Lithium Polycarboxylate-Nanoparticles. PCT/IB2020/053864 2020. Unpublished. [Google Scholar]
- Gargallo, L.; Radic, D.; Yazdani-Pedram, M.; Horta, A. Properties of polyelectrolytes: Poly(mono-methyl itaconate). conformational and viscometric behaviour in dilute solution. Eur. Polym. J. 1989, 25, 1059–1063. [Google Scholar] [CrossRef]
- Kellar, E.J.; Galiotis, C.; Andrews, E.H. Raman vibrational studies of syndiotactic polystyrene. 1. assignments in a conformational/crystallinity sensitive spectral region. Macromolecules 1996, 29, 3515–3520. [Google Scholar] [CrossRef]
- Rybachuk, M.; Hertwig, A.; Weise, M.; Sahre, M.; Männ, M.; Beck, U.; Bell, J.M. Near infrared optical materials from polymeric amorphous carbon synthesized by collisional plasma process. Appl. Phys. Lett. 2010, 96, 211909. [Google Scholar] [CrossRef] [Green Version]
- Dhahri, A.; Dhahri, E.; Hlil, E.K. Electrical conductivity and dielectric behaviour of nanocrystalline La0.6Gd0.1Sr0.3Mn0.75Si0.25O3. RSC Adv. 2018, 8, 9103–9111. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.K.; Liu, J.F.; Nowick, A.S. Limiting behavior of ac conductivity in ionically conducting crystals and glasses: A new universality. Phys. Rev. Lett. 1991, 67, 1559–1561. [Google Scholar] [CrossRef]
- Schönhals, A.; Kremer, F. Analysis of dielectric spectra. In Broadband Dielectric Spectroscopy; Kremer, F., Schönhals, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 978-3-642-56120-7. [Google Scholar]
- Billah, S.M.R. Dielectric polymers. In Functional Polymers; Jafar Mazumder, M.A., Sheardown, H., Al-Ahmed, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–49. ISBN 978-3-319-92067-2. [Google Scholar]
- Aguilar Bolados, H.; Hernández-Santana, M.; Romasanta, L.J.; Yazdani-Pedram, M.; Quijada, R.; López-Manchado, M.A.; Verdejo, R. Electro-mechanical actuation performance of SEBS/PU blends. Polymer 2019, 171, 25–33. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Vargas-Astudillo, D.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Fuentealba, P.; Contreras-Cid, A.; Verdejo, R.; López-Manchado, M.A. Facile and scalable one-step method for amination of graphene using leuckart reaction. Chem. Mater. 2017, 29. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Bolados, H.; Contreras-Cid, A.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Flores, M.; Fuentealba, P.; Neira-Carrillo, A.; Verdejo, R.; López-Manchado, M.A. Synthesis of fluorinated graphene oxide by using an easy one-pot deoxyfluorination reaction. J. Colloid Interface Sci. 2018. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Tian, M.; Yan, B.; Yao, Y.; Zhang, L.; Nishi, T.; Ning, N. High performance dielectric elastomers by partially reduced graphene oxide and disruption of hydrogen bonding of polyurethanes. Polymer 2015, 56, 375–384. [Google Scholar] [CrossRef]









| Sample | σ0 | A | s | R2 |
|---|---|---|---|---|
| PMMI | 6.4∙10−15 S cm−1 | 9.10∙10−15 | 0.971 | 0.993 |
| PMMI-Li+ | 4.8∙10−11 S cm−1 | 7.65∙10−13 | 0.784 | 0.999 |
| Sample | σ0 (S cm−1) | A | S | R2 | ε′ | tanδ | tanδmax |
|---|---|---|---|---|---|---|---|
| PMMI | 6.4∙10−15 | 9.10∙10−15 | 0.971 | 0.993 | 5.733 | 2.0∙10−2 | 2.0∙10−2 |
| PMMI-Li+ | 4.8∙10−11 | 7.65∙10−13 | 0.784 | 0.999 | 341.0 | 2.53∙100 | 2.53∙100 |
| PMMI/FGO (0.5 wt%) | 5.7∙10−14 | 3.45∙10−14 | 0.933 | 0.995 | 9.407 | 1.06∙10−1 | 1.06∙10−1 |
| PMMI/FGO (1.0 wt%) | 5.2∙10−14 | 3.39∙10−14 | 0.954 | 0.997 | 8.719 | 2.02∙10−1 | 2.02∙10−1 |
| PMMI/FGO (3.0 wt%) | 2.3∙10−14 | 3.05∙10−14 | 0.997 | 0.999 | 8.623 | 4.73∙10−2 | 4.73∙10−2 |
| PMMI/FGO (5.0 wt%) | 7.6∙10−14 | 3.23∙10−14 | 0.973 | 0.999 | 11.55 | 1.17∙10−1 | 1.17∙10−1 |
| PMMI-Li+/FGO (0.5 wt%) | 1.7∙10−10 | 7.59∙10−13 | 0.759 | 0.999 | 1175 | 2.60∙100 | 3.63∙100 |
| PMMI-Li+/FGO (1.0 wt%) | 7.8∙10−11 | 2.24∙10−13 | 0.824 | 0.999 | 409.2 | 3.48∙100 | 4.04∙100 |
| PMMI-Li+/FGO (3.0 wt%) | 5.6∙10−11 | 1.52∙10−12 | 0.702 | 0.997 | 339.1 | 2.99∙100 | 3.08∙100 |
| PMMI-Li+/FGO (5.0 wt%) | 2.3∙10−9 | 4.95∙10−12 | 0.728 | 0.993 | 7014 | 5.80∙100 | 6.33∙100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuenca-Bracamonte, Q.; Yazdani-Pedram, M.; Hernández Santana, M.; Aguilar-Bolados, H. Electrical Properties of Poly(Monomethyl Itaconate)/Few-Layer Functionalized Graphene Oxide/Lithium Ion Nanocomposites. Polymers 2020, 12, 2673. https://doi.org/10.3390/polym12112673
Cuenca-Bracamonte Q, Yazdani-Pedram M, Hernández Santana M, Aguilar-Bolados H. Electrical Properties of Poly(Monomethyl Itaconate)/Few-Layer Functionalized Graphene Oxide/Lithium Ion Nanocomposites. Polymers. 2020; 12(11):2673. https://doi.org/10.3390/polym12112673
Chicago/Turabian StyleCuenca-Bracamonte, Quimberly, Mehrdad Yazdani-Pedram, Marianella Hernández Santana, and Héctor Aguilar-Bolados. 2020. "Electrical Properties of Poly(Monomethyl Itaconate)/Few-Layer Functionalized Graphene Oxide/Lithium Ion Nanocomposites" Polymers 12, no. 11: 2673. https://doi.org/10.3390/polym12112673