Ibuprofen-Loaded Heparin Modified Thermosensitive Hydrogel for Inhibiting Excessive Inflammation and Promoting Wound Healing
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. PNIPAM-NH2 Synthesis
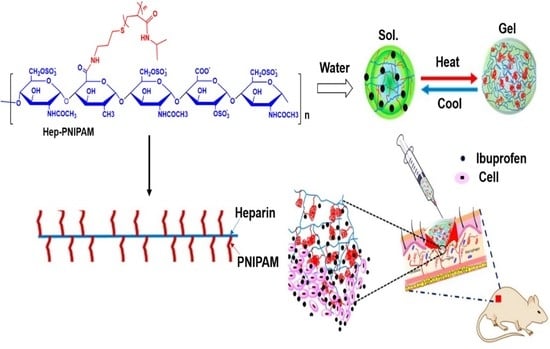
2.3. Heparin-PNIPAM Synthesis
2.4. Characterization of PNIPAM-NH2 and Hep-PNIPAM Copolymers
2.5. Grafting Efficiency and Ratio
2.6. Swelling Ratio
2.7. Hep-PNIPAM Copolymer Morphology
2.8. Lower Critical Solution Temperature (LCST) and Rheological Behavior
2.9. Ibuprofen Encapsulation and In Vitro Drug Release
2.10. In Vitro Cytotoxicity Test (MTT Assay)
2.11. Anti-Inflammation Assay for Ibuprofen-Loaded Hydrogel
2.11.1. Nitric Oxide (NO) Level Measurement
2.11.2. TNF-α and PGE2 Assays
2.12. Animal Experiment
2.12.1. Wound Healing Rate
2.12.2. In Vivo Anti-Inflammation Assay for Ibuprofen-Loaded Hydrogel
2.13. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Hep-PNIPAM Copolymer
3.2. Grafting Efficiency and Swelling Ratios
3.3. Lower Critical Solution Temperature (LCST) and Rheological Behavior
3.4. Ibuprofen Encapsulation and In Vitro Drug Release
3.5. In Vitro Cytotoxicity Test (MTT Assay)
3.6. Effects of IB-Hep-PNIPAM on NO, PGE2, and TNF-α Production in LPS-Stimulated RAW264.7 Cells
3.7. In Vivo Wound Healing Effects and Healing Rate
3.8. In Vivo Anti-Inflammation Assay on Ibuprofen-Loaded Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Martin, C.; Low, W.L.; Amin, M.C.I.M.; Radecka, I.; Raj, P.; Kenward, K. Current trends in the development of wound dressings, biomaterials and devices. Pharm. Pat. Anal. 2013, 2, 341–359. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Røine, E.; Bjørk, I.; Øyen, O. Targeting risk factors for impaired wound healing and wound complications after kidney transplantation. Transplant. Proc. 2010, 42, 2542–2546. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, J.; Zhu, W.; Yang, Z.; Li, B.; Yang, H.; Zheng, Q.; Cui, W. Ibuprofen-loaded electrospun fibrous scaffold doped with sodium bicarbonate for responsively inhibiting inflammation and promoting muscle wound healing in vivo. Biomater. Sci. 2014, 2, 502–511. [Google Scholar] [CrossRef]
- Woo, K.Y. Exploring the effects of pain and stress on wound healing. Adv. Ski. Wound Care 2012, 25, 38–44. [Google Scholar]
- Serena, T.E.; Yaakov, R.A.; Aslam, S. Preventing, minimizing, and managing pain in patients with chronic wounds: Challenges and solutions. Chronic Wound Care Manag. Res. 2016, 3, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar-Ricardo, A. Ibuprofen loaded PVA/chitosan membranes: A highly efficient strategy towards an improved skin wound healing. Carbohydr. Polym. 2017, 159, 136–145. [Google Scholar] [CrossRef]
- Sreij, R.; Prévost, S.; Dargel, C.; Dattani, R.; Hertle, Y.; Wrede, O.; Hellweg, T. Interaction of the Saponin Aescin with Ibuprofen in DMPC model membranes. Mol. Pharm. 2018, 15, 4446–4461. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Mucke, L. Ibuprofen, inflammation and Alzheimer disease. Nat. Med. 2000, 6, 973–974. [Google Scholar] [CrossRef]
- Bushra, R.; Aslam, N. An overview of clinical pharmacology of Ibuprofen. Oman Med. J. 2010, 25, 155–161. [Google Scholar] [CrossRef]
- Khalifa, N.; El-Husseini, T.; Morrah, A.; Mostafa, E.; Hamoud, H. Use of Ibuprofen sustained release for treating osteoarthritic pain: Findings from 15 general medical practices in Egypt. Open Access Rheumatol. Res. Rev. 2014, 6, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Bian, Z.; Tang, J.; Hu, J.; Li, J.; Xu, S.; Liu, H. Controlled-release of ibuprofen on multilayer mesoporous vesicle. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 1021–1026. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.C.; Lai, J.Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Hanurry, E.Y.; Birhan, Y.S.; Mekonnen, T.W.; Chou, H.Y.; Hsu, W.H.; Lai, J.Y.; Lin, S.Y.; Tsai, H.C. Localized controlled release of bevacizumab and doxorubicin by thermo-sensitive hydrogel for normalization of tumor vasculature and to enhance the efficacy of chemotherapy. Int. J. Pharm. 2019, 572, 118799. [Google Scholar] [CrossRef]
- Ma, H.; He, C.; Cheng, Y.; Yang, Z.; Zang, J.; Liu, J.; Chen, X. Localized Co-delivery of Doxorubicin, Cisplatin, and Methotrexate by thermosensitive hydrogels for enhanced Osteosarcoma treatment. ACS Appl. Mater. Interfaces 2015, 7, 27040–27048. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Hirakura, T.; Sakurai, Y.; Shimoboji, T.; Ishigai, M.; Akiyoshi, K. Injectable hydrogel for sustained protein release by salt-induced association of hyaluronic acid nanogel. Macromol. Biosci. 2012, 12, 475–483. [Google Scholar] [CrossRef]
- Andrgie, A.T.; Mekuria, S.L.; Addisu, K.D.; Hailemeskel, B.Z.; Hsu, W.; Tsai, H.; Lai, J. Non-anticoagulant heparin prodrug loaded biodegradable and injectable thermoresponsive hydrogels for enhanced anti-metastasis therapy. Macromol. Biosci. 2019, 19, e1800409. [Google Scholar] [CrossRef]
- Jin, S.G.; Yousaf, A.M.; Kim, K.S.; Kim, D.S.; Kim, J.K.; Yong, C.S.; Youn, Y.S.; Kim, J.O.; Choi, H.G. Influence of hydrophilic polymers on functional properties and wound healing efficacy of hydrocolloid based wound dressings. Int. J. Pharm. 2016, 501, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.Z.; West, Z.E.; Cowin, A.J.; Farrugia, B.L. Development and use of biomaterials as wound healing therapies. Burn. Trauma 2019, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.G.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Tay, F.R. Recent progress in the industrial and biomedical applications of tragacanth gum: A review. Carbohydr. Polym. 2019, 212, 450–467. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Borzacchiello, A.; Tay, F.R.; Ashtari, K.; Padil, V.V.T. Antimicrobial gum bio-based nanocomposites and their industrial and biomedical applications. Chem. Commun. 2019, 55, 14871–14885. [Google Scholar] [CrossRef]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 1–22. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef]
- Jiang, G.; Sun, J.; Ding, F. PEG-g-chitosan thermosensitive hydrogel for implant drug delivery: Cytotoxicity, in vivodegradation and drug release. J. Biomater. Sci. Polym. Ed. 2013, 25, 241–256. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, C.; Li, X.; Xu, C.; Zhang, Y.; Sun, Z.; Liu, Y.; Gao, J. Thermosensitive methyl cellulose-based injectable hydrogels for post-operation anti-adhesion. Carbohydr. Polym. 2014, 101, 171–178. [Google Scholar] [CrossRef]
- Goh, M.; Hwang, Y.; Sahu, A. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef]
- Roberts, J.J.; Farrugia, B.L.; Green, R.A.; Rnjak-Kovacina, J.; Martens, P.J. In situ formation of poly(vinyl alcohol)–heparin hydrogels for mild encapsulation and prolonged release of basic fibroblast growth factor and vascular endothelial growth factor. J. Tissue Eng. 2016, 7, 2041731416677132. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, Z.; Guo, L.; Ye, L.; Tong, Z.; Geng, X.; Wang, C.; Jin, X.; Hui, X.; Gu, Y. Biocompatibility evaluation of heparin-conjugated poly(ε-caprolactone) scaffolds in a rat subcutaneous implantation model. J. Mater. Sci. Mater. Med. 2020, 31, 1–13. [Google Scholar] [CrossRef]
- Ikegami, Y.; Ijima, H. Development of heparin-conjugated nanofibers and a novel biological signal by immobilized growth factors for peripheral nerve regeneration. J. Biosci. Bioeng. 2020, 129, 354–362. [Google Scholar] [CrossRef] [PubMed]
- La, W.G.; Yang, H.S. Heparin-conjugated poly(Lactic-Co-Glycolic Acid) nanospheres enhance large-wound healing by delivering growth factors in platelet-rich plasma. Artif. Organs 2014, 39, 388–394. [Google Scholar] [CrossRef]
- Uzunalli, G.; Mammadov, R.; Yesildal, F.; Alhan, D.; Ozturk, S.; Özgürtaş, T.; Guler, M.O.; Tekinay, A.B. Angiogenic heparin-mimetic peptide nanofiber gel improves regenerative healing of acute wounds. ACS Biomater. Sci. Eng. 2016, 3, 1296–1303. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-based and heparin-inspired hydrogels: Size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef]
- Du, L.; Tong, L.; Jin, Y.; Jia, J.; Liu, Y.; Su, C.; Yu, S.; Li, X. A multifunctional in situ-forming hydrogel for wound healing. Wound Repair Regen. 2012, 20, 904–910. [Google Scholar] [CrossRef]
- Hoque, J.; Prakash, R.G.; Paramanandham, K.; Shome, B.R.; Haldar, J. Biocompatible injectable hydrogel with potent wound healing and antibacterial properties. Mol. Pharm. 2017, 14, 1218–1230. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Zhou, J.; Yan, J.; Wang, D.; Zhang, H. Redox-responsive tumor targeted dual-drug loaded biocompatible metal–organic frameworks nanoparticles for enhancing anticancer effects. BMC Mater. 2020, 2, 1–11. [Google Scholar]
- Bubpamala, T.; Viravaidya-Pasuwat, K.; Pholpabu, P. Injectable poly(ethylene glycol) hydrogels cross-linked by metal–phenolic complex and albumin for controlled drug release. ACS Omega 2020, 5, 19437–19445. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, M.; Wu, L.; Pan, S.; Zhang, Y.; He, B.; He, P. Physically cross-linked double-network hydrogel for high-performance oil–water separation mesh. Ind. Eng. Chem. Res. 2019, 58, 21649–21658. [Google Scholar] [CrossRef]
- Chen, W.; Tian, X.; He, W.; Li, J.; Feng, Y.; Pan, G. Emerging functional materials based on chemically designed molecular recognition. BMC Mater. 2020, 2, 1–22. [Google Scholar] [CrossRef]
- Mi, L.; Xue, H.; Li, Y.; Jiang, S. A Thermoresponsive antimicrobial wound dressing hydrogel based on a cationic Betaine ester. Adv. Funct. Mater. 2011, 21, 4028–4034. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Alexander, A.; Khan, J.; Saraf, S.; Saraf, S. Polyethylene glycol (PEG)–poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef]
- Chen, J.P.; Cheng, T.H. Thermo-responsive Chitosan-graft-poly(N-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromol. Biosci. 2006, 6, 1026–1039. [Google Scholar] [CrossRef]
- Ohya, S.; Nakayama, Y.; Matsuda, T. In vivo evaluation of poly(N-isopropylacrylamide) (PNIPAM)-grafted gelatin as an in situ-formable scaffold. J. Artif. Organs 2005, 7, 181–186. [Google Scholar] [CrossRef]
- Antoniraj, M.G.; Kumar, C.S.; Kandasamy, R. Synthesis and characterization of poly(N-isopropylacrylamide)-g-carboxymethyl chitosan copolymer-based doxorubicin-loaded polymeric nanoparticles for thermoresponsive drug release. Colloid Polym. Sci. 2015, 294, 527–535. [Google Scholar] [CrossRef]
- Xing, R.; Li, S.; Zhang, N.; Shen, G.; Möhwald, H.; Yan, X. Self-assembled injectable Peptide hydrogels capable of triggering antitumor immune response. Biomacromolecules 2017, 18, 3514–3523. [Google Scholar] [CrossRef]
- Lee, E.; Kim, D.; Kim, H.; Yoon, J. Photothermally driven fast responding photo-actuators fabricated with comb-type hydrogels and magnetite nanoparticles. Sci. Rep. 2015, 5, 15124. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Kim, D.; Yang, S.Y.; Oh, J.-W.; Yoon, J. Photo-crosslinkable comb-type copolymers bearing a benzophenone moiety for the enhanced swelling kinetics of hydrogels. Polym. Chem. 2017, 8, 6786–6794. [Google Scholar] [CrossRef]
- Muramatsu, K.; Ide, M.; Miyawaki, F. Biological evaluation of tissue-engineered cartilage using thermoresponsive poly(N-isopropylacrylamide)-grafted hyaluronan. J. Biomater. Nanobiotechnol. 2012, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Darge, H.F.; Hanurry, E.Y.; Birhan, Y.S.; Mekonnen, T.W.; Andrgie, A.T.; Chou, H.Y.; Lai, J.Y.; Tsai, H.C. Multifunctional drug-loaded micelles encapsulated in thermo-sensitive hydrogel for in vivo local cancer treatment: Synergistic effects of anti-vascular and immuno-chemotherapy. Chem. Eng. J. 2020, 406, 126879. [Google Scholar] [CrossRef]
- Chen, J.P.; Leu, Y.L.; Fang, C.L.; Chen, C.H.; Fang, J.Y. Thermosensitive hydrogels composed of hyaluronic acid and gelatin as carriers for the intravesical administration of cisplatin. J. Pharm. Sci. 2011, 100, 655–666. [Google Scholar] [CrossRef]
- Addisu, K.D.; Hailemeskel, B.Z.; Mekuria, S.L.; Andrgie, A.T.; Lin, Y.C.; Tsai, H.C. Bioinspired, manganese-chelated alginate–Polydopamine nanomaterials for efficient in vivo T1-weighted magnetic resonance imaging. ACS Appl. Mater. Interfaces 2018, 10, 5147–5160. [Google Scholar] [CrossRef]
- Birhan, Y.S.; Hailemeskel, B.Z.; Mekonnen, T.W.; Hanurry, E.Y.; Darge, H.F.; Andrgie, A.T.; Chou, H.Y.; Lai, J.Y.; Hsiue, G.-H.; Tsai, H.C. Fabrication of redox-responsive Bi(mPEG-PLGA)-Se2 micelles for doxorubicin delivery. Int. J. Pharm. 2019, 567, 118486. [Google Scholar] [CrossRef]
- Hailemeskel, B.Z.; Hsu, W.H.; Addisu, K.D.; Andrgie, A.T.; Chou, H.Y.; Lai, J.Y.; Tsai, H.C. Diselenide linkage containing triblock copolymer nanoparticles based on Bi(methoxyl poly(ethylene glycol))-poly(ε-carprolactone): Selective intracellular drug delivery in cancer cells. Mater. Sci. Eng. C 2019, 103, 109803. [Google Scholar] [CrossRef]
- Nguyen, L.; Truong, C.T.; Nguyen, B.C.Q.; Vo, N.; Dao, T.T.; Nguyen, V.D.; Trinh, D.T.T.; Huynh, H.K.; Bui, C.B. Anti-inflammatory and wound healing activities of calophyllolide isolated from Calophyllum inophyllum Linn. PLoS ONE 2017, 12, e0185674. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.R.; Ghute, P.P.; Badiger, M.V. Synthesis and characterization of thermo-sensitive graft copolymer of carboxymethyl guar and poly(N-isopropylacrylamide). Carbohydr. Polym. 2011, 83, 74–80. [Google Scholar] [CrossRef]
- Qi, M.; Li, G.; Yu, N.; Meng, Y.; Liu, X. Synthesis of thermo-sensitive polyelectrolyte complex nanoparticles from CS-g-PNIPAM and SA-g-PNIPAM for controlled drug release. Macromol. Res. 2014, 22, 1004–1011. [Google Scholar] [CrossRef]
- Liu, K.; Pan, P.; Bao, Y. Synthesis, micellization, and thermally-induced macroscopic micelle aggregation of poly(vinyl chloride)-g-poly(N-isopropylacrylamide) amphiphilic copolymer. RSC Adv. 2015, 5, 94582–94590. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Lopez, F. Rheological Characterization of Hydrogels from Alginate-Based Nanodispersion. Polymers 2019, 11, 259. [Google Scholar] [CrossRef] [Green Version]
- Pramod, K.; Shanavas, S.; Baby, I.N. Rheological profiling of a hydrogel drug delivery vehicle. J. Chem. Pharm. Res. 2015, 7, 818–825. [Google Scholar]
- Tie, L.; Yu, M.; Li, X.; Liu, W.; Zhang, B.; Chang, Z.; Zheng, Y. Research on polymer solution rheology in polymer flooding for Qikou reservoirs in a Bohai Bay oilfield. J. Pet. Explor. Prod. Technol. 2018, 9, 703–715. [Google Scholar] [CrossRef] [Green Version]
- Redpath, M.; Marques, C.; Dibden, C.; Waddon, A.; Lalla, R.; MacNeil, S. Ibuprofen and hydrogel-released ibuprofen in the reduction of inflammation-induced migration in melanoma cells. Br. J. Dermatol. 2009, 161, 25–33. [Google Scholar] [CrossRef]
- Pham, C.V.; Van, M.C.; Thi, H.P.; Thanh, C.Đ.; Ngoc, B.T.; Van, B.N.; Le Thien, G.; Nguyen, C.N. Development of ibuprofen-loaded solid lipid nanoparticle-based hydrogels for enhanced in vitro dermal permeation and in vivo topical anti-inflammatory activity. J. Drug Deliv. Sci. Technol. 2020, 57, 101758. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, K.J.; Ryu, S.J.; Lee, B.Y. Caffeine prevents LPS-induced inflammatory responses in RAW264.7 cells and zebrafish. Chem. Interact. 2016, 248, 1–7. [Google Scholar] [CrossRef]
- Cao, S.Y.; Wang, W.; Nan, F.F.; Liu, Y.N.; Wei, S.Y.; Li, F.F.; Chen, L. Asiatic acid inhibits LPS-induced inflammatory response in endometrial epithelial cells. Microb. Pathog. 2018, 116, 195–199. [Google Scholar] [CrossRef]
- Liao, W.; He, X.; Yi, Z.; Xiang, W.; Ding, Y. Chelidonine suppresses LPS-Induced production of inflammatory mediators through the inhibitory of the TLR4/NF-κB signaling pathway in RAW264. 7 macrophages. Biomed. Pharmacother. 2018, 107, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhi, M.; Feng, Z.; Gao, P.; Yuan, Y.; Zhang, C.; Wang, Y.; Dong, A. Sustained co-delivery of ibuprofen and basic fibroblast growth factor by thermosensitive nanoparticle hydrogel as early local treatment of peri-implantitis. Int. J. Nanomed. 2019, 14, 1347–1358. [Google Scholar] [CrossRef] [Green Version]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 2015, 49, 37–46. [Google Scholar] [CrossRef]










| Polymer | Heparin (g) | PNIPAM-NH2 (g) | Grafting Ratio | Yield (%) | Swelling Ratio |
|---|---|---|---|---|---|
| Hep-PNIPAM-b1 | 0.1 | 1.5 | 10 | 70 | 22.5 ± 2.9 |
| Hep-PNIPAM-b2 | 0.1 | 2 | 17 | 96 | 14.2 ± 1.1 |
| Hep-PNIPAM-b3 | 0.1 | 3 | 23 | 84 | 12.3 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrgie, A.T.; Darge, H.F.; Mekonnen, T.W.; Birhan, Y.S.; Hanurry, E.Y.; Chou, H.-Y.; Wang, C.-F.; Tsai, H.-C.; Yang, J.M.; Chang, Y.-H. Ibuprofen-Loaded Heparin Modified Thermosensitive Hydrogel for Inhibiting Excessive Inflammation and Promoting Wound Healing. Polymers 2020, 12, 2619. https://doi.org/10.3390/polym12112619
Andrgie AT, Darge HF, Mekonnen TW, Birhan YS, Hanurry EY, Chou H-Y, Wang C-F, Tsai H-C, Yang JM, Chang Y-H. Ibuprofen-Loaded Heparin Modified Thermosensitive Hydrogel for Inhibiting Excessive Inflammation and Promoting Wound Healing. Polymers. 2020; 12(11):2619. https://doi.org/10.3390/polym12112619
Chicago/Turabian StyleAndrgie, Abegaz Tizazu, Haile Fentahun Darge, Tefera Worku Mekonnen, Yihenew Simegniew Birhan, Endiries Yibru Hanurry, Hsiao-Ying Chou, Chih-Feng Wang, Hsieh-Chih Tsai, Jen Ming Yang, and Yen-Hsiang Chang. 2020. "Ibuprofen-Loaded Heparin Modified Thermosensitive Hydrogel for Inhibiting Excessive Inflammation and Promoting Wound Healing" Polymers 12, no. 11: 2619. https://doi.org/10.3390/polym12112619