Biopolymer Hydrogel Scaffold as an Artificial Cell Niche for Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Plasma
2.2. Cell Cultures
2.3. Hydrogel Scaffold Formation
2.4. Scanning Electronic Microscopy
2.5. Comparative Characteristics of the Porosity of the Structure of Scaffolds
2.6. Fluorescence Microscopy
2.7. Quantitative Analysis of Cells in Scaffolds
2.8. Quantification of VEGF in the Growth Medium
2.9. Scaffold Biodegradation Study
2.10. Statistical Analysis
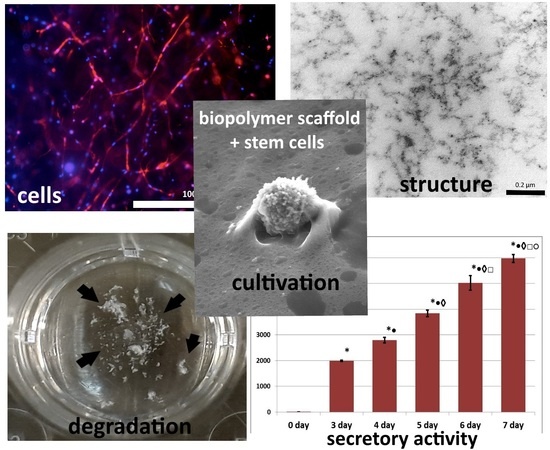
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krishna, L.; Dhamodaran, K.; Jayadev, C.; Chatterjee, K.; Shetty, R.; Khora, S.S.; Das, D. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res. Therapy 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drela, K.; Stanaszek, L.; Nowakowski, A.; Kuczynska, Z.; Lukomska, B. Experimental Strategies of Mesenchymal Stem Cell Propagation: Adverse Events and Potential Risk of Functional Changes. Stem Cells Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.R.; Yong, K.W.; Nam, H.Y. Current status and perspectives of human mesenchymal stem cell therapy. Stem Cells Int. 2019, 2019, 4762634. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmon, C.A. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018, 2018, 4762634. [Google Scholar] [CrossRef]
- Sávio-Silva, C.; Soinski-Sousa, P.E.; Balby-Rocha, M.T.A.; Lira, Á.D.O.; Rangel, É.B. Mesenchymal stem cell therapy in acute kidney injury (AKI): Review and perspectives. Revista da Associacao Medica Brasileira 2020, 66, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Regmi, S.; Pathak, S.; Kim, J.O.; Yong, C.S.; Jeong, J.-H. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. Eur. J. Cell Biol. 2019, 98, 151041. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/home (accessed on 29 October 2020).
- Gomez-Salazar, M.; Gonzalez-Galofre, Z.N.; Casamitjana, J.; Crisan, M.; James, A.W.; Péault, B. Five Decades Later, Are Mesenchymal Stem Cells Still Relevant? Front. Bioeng. Biotechnol. 2020, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Taddei, M.L.; Giannoni, E.; Fiaschi, T.; Chiarugi, P. Anoikis: An emerging hallmark in health and diseases. J. Pathol. 2012, 226, 380–393. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Pennings, S.; Liu, K.J.; Qian, H. The stem cell niche: Interactions between stem cells and their environment. Stem Cells Inter. 2018, 2018, 4879379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, F.M.; Huck, W.T.S. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013, 14, 467–473. [Google Scholar] [CrossRef]
- Pardo-Saganta, A.; Calvo, I.A.; Saez, B.; Prosper, F. Role of the Extracellular Matrix in Stem Cell Maintenance. Curr. Stem Cell Rep. 2019, 5, 1. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, C.; Zhang, P.; Jiang, H.; Chen, J. Preconditioning strategies for improving the survival rate and paracrine ability of mesenchymal stem cells in acute kidney injury. J. Cell. Mol. Med. 2019, 23, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Laurencin, C.; Lu, H.; Williams, D.; Simon, C.; Lu, H.; Best, S. Regenerative Medicine in Definitions of Biomaterials for the Twenty-First Century; Williams, D., Zhang, X., Eds.; Elsevier Inc.: New York, NY, USA, 2019; pp. 115–153. [Google Scholar]
- Zhang, X.D.; Williams, D.F. Definitions of Biomaterials for the Twenty-first Century. In Proceedings of the Consensus Conference on Definitions of Biomaterials for the Twenty-First Century, Chengdu, China, 11–12 June 2018. [Google Scholar]
- Williams, D.F. Challenges with the development of biomaterials for sustainable tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Akhmanova, M.; Osidak, E.; Domogatsky, S.; Rodin, S.; Domogatskaya, A. Physical, Spatial, and Molecular Aspects of Extracellular Matrix of in Vivo Niches and Artificial Scaffolds Relevant to Stem Cells Research. Stem Cells Int. 2015, 2015, 167025. [Google Scholar] [CrossRef] [Green Version]
- Smith, Q.; Gerecht, S. Extracellular Matrix Regulation of Stem Cell Fate. Curr. Stem Cell Rep. 2018, 4, 13–21. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, S.; Liu, N.; Li, Z. Extracellular Matrix Enhances Therapeutic Effects of Stem Cells in Regenerative Medicine. Compos. Funct. Extracell. Matrix Human Body 2016, 323–340. [Google Scholar]
- Sharma, M.; Ross, C.; Srivastava, S. Ally to adversary: Mesenchymal stem cells and their transformation in leukaemia. Cancer Cell Inter. BioMed Central 2019, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.J.; Kershner, A.M.; Beachy, P.A. The Stromal Niche for Epithelial Stem Cells: A Template for Regeneration and a Brake on Malignancy. Cancer Cell 2017, 32, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubert, L.; Dubus, M.; Rammal, H.; Bour, C.; Mongaret, C.; Boulagnon-Rombi, C.; Garnotel, R.; Schneider, C.; Rahouadj, R.; Laurent, C.; et al. Collagen-based medical device as a stem cell carrier for regenerative medicine. Int. J. Mol. Sci. 2017, 18, 2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, K.H.; Kim, Y.; Song, S. Fine-Tunable and Injectable 3D Hydrogel for On-Demand Stem Cell Niche. Adv. Sci. 2019, 6, 1900597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egorikhina, M.N.; Aleynik, D.Y.; Rubtsova, Y.P.; Levin, G.Y.; Charykova, I.N.; Semenycheva, L.L.; Bugrova, M.L.; Zakharychev, E.A. Hydrogel scaffolds based on blood plasma cryoprecipitate and collagen derived from various sources: Structural, mechanical and biological characteristics. Bioact. Mater. 2019, 4, 334–345. [Google Scholar] [CrossRef]
- Dominici, M.; Blanc, K.L.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Levin, G.Y.; Charykova, I.N.; Alejnik, D.Y.; Sosnina, L.N. Method for Creating a Bioresorbable Cellular Scaffold Based on Fibrin of Blood Plasma. Int. Cl. C12N 5/00 Bull. Patent No. 653434 RU, 8 May 2018. [Google Scholar]
- Semenycheva, L.L.; Astanina, M.V.; Kuznetsova, J.L.; Valetova, N.B.; Geras’kina, E.V.; Tarankova, O.A. Method for Production of Acetic Dispersion of High Molecular Fish Collagen. Int. Cl. C08H 1/06, A23J 1/04 Bull. Patent 2567171, 10 November 2015. [Google Scholar]
- Semenycheva, L.L.; Egorikhina, M.N.; Chasova, V.O.; Valetova, N.B.; Kuznetsova, Y.L.; Mitin, A.V. Enzymatic hydrolysis of marine collagen and fibrinogen proteins in the presence of thrombin. Mar. Drugs 2020, 18, 208. [Google Scholar] [CrossRef] [Green Version]
- Egorikhina, M.N.; Charykova, I.N.; Alejnik, D.Y. Method of Quantitative Analysis of Cellular Components of Scaffold. Int. Cl. G01N 33/52 Bull. Patent No. 2675376 RU, 19 December 2018. [Google Scholar]
- Sharma, V.; Patel, N.; Kohli, N.; Ravindran, N.; Hook, L.; Mason, C.; García-Gareta, E. Viscoelastic, physical, and bio-degradable properties of dermal scaffolds and related cell behaviour. Biomed. Mater. 2016, 11, 055001. [Google Scholar] [CrossRef]
- Liddington, R.C.; Ginsberg, M.H. Integrin activation takes shape. J. Cell Biol. 2002, 158, 833–839. [Google Scholar] [CrossRef]
- Prowse, A.B.J.; Chong, F.; Gray, P.P.; Munro, T.P. Stem cell integrins: Implications for ex-vivo culture and cellular therapies. Stem Cell Res. 2011, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kaijzel, E.L.; Koolwijk, P.; van Erck, M.G.; van Hinsbergh, V.W.; de Maat, M.P. Molecular weight fibrinogen variants determine angiogenesis rate in a fibrin matrix in vitro and in vivo. J. Thromb. Haemost. 2006, 4, 1975–1981. [Google Scholar] [CrossRef]
- Laurens, N.; Engelse, M.A.; Jungerius, C.; Löwik, C.W.; van Hinsbergh, V.W.; Koolwijk, P. Single and combined effects of alphavbeta3- and alpha5beta1-integrins on capillary tube formation in a human fibrinous matrix. Angiogenesis 2009, 12, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mork, B.C.; Vitebsky, A.; Hou, M.; Preising, B. How the Pink or Blue® DNA Gender Test Works. October 2008, 32, 2–5. [Google Scholar]
- Ames, J.J.; Contois, L.; Caron, J.M.; Tweedie, E.; Yang, X.; Friesel, R.; Vary, C.; Brooks, P.C. Identification of an endogenously generated cryptic collagen epitope (XL313) that may selectively regulate angiogenesis by an integrin yes-associated protein (YAP) mechano-transduction pathway. J. Biol. Chem. 2016, 291, 2731–2750. [Google Scholar] [CrossRef] [Green Version]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J.; Liddington, R.C. Structural Basis of Collagen Recognition by Integrin α2β1. Cell 2000, 101, 47–56. [Google Scholar] [CrossRef]
- Knight, C.G.; Morton, L.F.; Peachey, A.R.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. The collagen-binding a-domains of integrins α1/β1 and α2/β1 recognize the same specific amino acid sequence, GFOGER, in native (triple- helical) collagens. J. Biol. Chem. 2000, 275, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, B.-H.; Carman, C.V.; Springer, T.A. Structural Basis of Integrin Regulation and Signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [Green Version]
- To, W.S.; Midwood, K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogen. Tissue Rep. 2011, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Sparrow, R.L.; Simpson, R.J.; Greening, D.W. A Protocol for the Preparation of Cryoprecipitate and Cryo-depleted Plasma for Proteomic Studies. Methods Mol. Biol. 2017, 1619, 23–30. [Google Scholar]
- Nascimento, B.; Goodnough, L.T.; Levy, J.H. Cryoprecipitate therapy. Brit. J. Anaesth. 2014, 113, 922–934. [Google Scholar] [CrossRef] [Green Version]
- Hynes, R.O. The extracellu. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Sahni, A.; Guo, M.; Sahni, S.K.; Francis, C.W. Interleukin-1β but not IL-1α binds to fibrinogen and fibrin and has enhanced activity in the bound form. Blood 2004, 104, 409–414. [Google Scholar] [CrossRef]
- Martino, M.M.; Briquez, P.S.; Ranga, A.; Lutolf, M.P.; Hubbell, J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proceedings of the National Academy of Sciences of the United States of America. Soc. Exploit. Vitellogen. 2013, 110, 4563–4568. [Google Scholar]
- Jeon, O.; Ryu, S.H.; Chung, J.H.; Kim, B.-S. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J. Control. Release 2005, 105, 249–259. [Google Scholar] [CrossRef]
- Briganti, E.; Spiller, D.; Mirtelli, C.; Kull, S.; Counoupas, C.; Losi, P.; Senesi, S.; Di Stefano, R.; Soldani, G. A composite fibrin-based scaffold for controlled delivery of bioactive pro-angiogenetic growth factors. J. Control. Release 2010, 142, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Panetti, T.S.; Kudryk, B.J.; Mosher, D.F. Interaction of recombinant procollagen and properdin modules of thrombospondin-1 with heparin and fibrinogen/fibrin. J. Biol. Chem. 1999, 274, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjipanayi, E.; Kuhn, P.-H.; Moog, P.; Bauer, A.-T.; Kuekrek, H.; Mirzoyan, L.; Hummel, A.; Kirchhoff, K.; Salgin, B.; Isenburg, S.; et al. The fibrin matrix regulates angiogenic responses within the hemostatic microenvironment through biochemical control. PLoS ONE 2015, 10, e0135618. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Ataabadi, M.; Mostafavi-pour, Z.; Vojdani, Z.; Sani, M.; Latifi, M.; Talaei-Khozani, T. Fabrication and characterization of platelet-rich plasma scaffolds for tissue engineering applications. Mater. Sci. Eng. 2017, 71, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Wang, D.-A. Macroporous Hydrogel Scaffolds for Three-Dimensional Cell Culture and Tissue Engineering. Tissue Eng. Part B Rev. 2017, 23, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohmann, T.; Dehghani, F. The Cytoskeleton—A Complex Interacting Meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.D.; Gerlach, B.D. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Resp. Res. 2017, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular mechanotransduction: From tension to function. Front. Physiol. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Y.; Fu, J. Microfabricated Nanotopological Surfaces for Study of Adhesion-dependent Cell mechanosensitivity. Small 2013, 9, 81–89. [Google Scholar] [CrossRef]
- Argentati, C.; Morena, F.; Tortorella, I.; Bazzucchi, M.; Porcellati, S.; Emiliani, C.; Martino, S. Insight into mechanobiology: How stem cells feel mechanical forces and orchestrate biological functions. Int. J. Sci. 2019, 20, 5337. [Google Scholar] [CrossRef] [Green Version]
- Shannalee, R.; Maresha, S.M.; Gay, L.Z. Revisiting the matricellular concept. Physiol. Behav. 2016, 176, 139–148. [Google Scholar]
- Aleynik, D.Y.; Zagaynova, E.V.; Egorikhina, M.N.; Charykova, I.N.; Rogovaya, O.S.; Rubtsova, Y.P.; Popova, A.N.; Vorotelyak, E.A. Methods for Assessing the Quality of Biomedical Cell Products for Skin Replacement. Sovremennye Tehnologii v Med. 2019, 11, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.P.; González, M.; Ríos, S.; Cambiazo, V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J. Cell. Biochem. 2004, 93, 721–731. [Google Scholar] [CrossRef]
- Saidova, A.A.; Vorobjev, I.A. Lineage Commitment, Signaling Pathways, and the Cytoskeleton Systems in Mesenchymal Stem Cells. Tissue Eng. Part B Rev. 2020, 26, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.L.; Goldmann, W.H. Cellular mechanotransduction. AIMS Biophys. 2016, 3, 50–62. [Google Scholar] [CrossRef]
- Karaman, S.; Leppänen, V.M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, M.; Wu, Y.; Danaoui, Y.; Ghosh, G. Engineering microenvironments towards harnessing pro-angiogenic potential of mesenchymal stem cells. Mater. Sci. Eng. C 2019, 102, 75–84. [Google Scholar] [CrossRef]
- Assi, R.; Foster, T.R.; He, H.; Stamati, K.; Bai, H.; Huang, Y.; Hyder, F.; Rothman, D.; Shu, C.; Homer-Vanniasinkam, S.; et al. Delivery of mesenchymal stem cells in biomimetic engineered scaffolds promotes healing of diabetic ulcers. Regen. Med. 2016, 11, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Therapy 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]







| Cultivation | Total Number of Cell Nuclei per 1 mm3 Scaffold | Number of Nuclei of Dead Cells per 1 mm3 Scaffold | % of Nuclei of Dead Cells |
|---|---|---|---|
| 72 h | 292.07 ± 15.51 | 2.33 ± 0.31 | 0.79 |
| 144 h | 636.79 ± 33.85 | 3.60 ± 0.42 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorikhina, M.N.; Rubtsova, Y.P.; Charykova, I.N.; Bugrova, M.L.; Bronnikova, I.I.; Mukhina, P.A.; Sosnina, L.N.; Aleynik, D.Y. Biopolymer Hydrogel Scaffold as an Artificial Cell Niche for Mesenchymal Stem Cells. Polymers 2020, 12, 2550. https://doi.org/10.3390/polym12112550
Egorikhina MN, Rubtsova YP, Charykova IN, Bugrova ML, Bronnikova II, Mukhina PA, Sosnina LN, Aleynik DY. Biopolymer Hydrogel Scaffold as an Artificial Cell Niche for Mesenchymal Stem Cells. Polymers. 2020; 12(11):2550. https://doi.org/10.3390/polym12112550
Chicago/Turabian StyleEgorikhina, Marfa N., Yulia P. Rubtsova, Irina N. Charykova, Marina L. Bugrova, Irina I. Bronnikova, Polina A. Mukhina, Larisa N. Sosnina, and Diana Ya. Aleynik. 2020. "Biopolymer Hydrogel Scaffold as an Artificial Cell Niche for Mesenchymal Stem Cells" Polymers 12, no. 11: 2550. https://doi.org/10.3390/polym12112550