Poly (Dimethylsiloxane) Coating for Repellency of Polar and Non-Polar Liquids
Abstract
:1. Introduction
2. Experimental
2.1. Materials
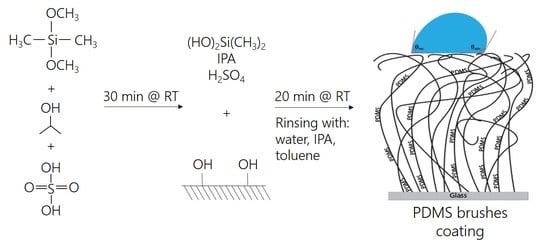
2.2. Preparation of PDMS Brush Coating
2.3. Contact Angle Measurements
2.4. Atomic Force Microscopy
3. Results and Discussion
3.1. Wettability of Polar and Non-Polar Liquids
3.2. Sliding Effect of Non-Polar Liquids
3.3. Comprehensive Modeling of CAH
3.4. Surface Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired surfaces with superwettability: New insight on theory, design, and applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Tian, Y.; Jiang, L. Bioinspired super-wettability from fundamental research to practical applications. Angew. Chem. Int. Ed. 2015, 54, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tian, Y.; Jiang, L. Bio-inspired superoleophobic and smart materials: Design, fabrication, and application. Prog. Mater. Sci. 2013, 58, 503–564. [Google Scholar] [CrossRef]
- Wooh, S.; Vollmer, D. Silicone Brushes: Omniphobic Surfaces with Low Sliding Angles. Angew. Chem. Int. Ed. 2016, 55, 6822–6824. [Google Scholar] [CrossRef]
- Bracco, G.; Holst, B. (Eds.) Surface Science Techniques; Springer: Berlin, Germany, 2013; ISBN 978-3-642-34242-4. [Google Scholar]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Huhtamäki, T.; Ikkala, O.; Ras, R.H.A. Reliable measurement of the receding contact angle-supporting information. Langmuir 2013, 29, 3858–3863. [Google Scholar] [CrossRef] [PubMed]
- Urata, C.; Masheder, B.; Cheng, D.F.; Miranda, D.F.; Dunderdale, G.J.; Miyamae, T.; Hozumi, A. Why can organic liquids move easily on smooth alkyl-terminated surfaces? Langmuir 2014, 30, 4049–4055. [Google Scholar] [CrossRef]
- Boban, M.; Golovin, K.; Tobelmann, B.; Gupte, O.; Mabry, J.M.; Tuteja, A. Smooth, All-Solid, Low-Hysteresis, Omniphobic Surfaces with Enhanced Mechanical Durability. ACS Appl. Mater. Interfaces 2018, 10, 11406–11413. [Google Scholar] [CrossRef]
- Eral, H.B.; ’t Mannetje, D.J.C.M.; Oh, J.M. Contact angle hysteresis: A review of fundamentals and applications. Colloid Polym. Sci. 2013, 291, 247–260. [Google Scholar] [CrossRef]
- Darmanin, T.; Taffin de Givenchy, E.; Guittard, F. Chemical and Physical Pathways for the Preparation of Superoleophobic Surfaces and Related Wetting Theories. Chem. Rev. 2014, 114, 2694–2716. [Google Scholar]
- Jiang, T.; Guo, Z.; Liu, W. Biomimetic superoleophobic surfaces: Focusing on their fabrication and applications. Mater. Chem. A 2015, 3, 1811–1827. [Google Scholar] [CrossRef]
- Zhao, H.; Law, K.Y.; Sambhy, V. Fabrication, surface properties, and origin of superoleophobicity for a model textured surface. Langmuir 2011, 27, 5927–5935. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Lim, J.I.; Lee, B.R.; Mun, C.H.; Jung, Y.; Kim, S.H. Preparation of lotus-leaf-like structured blood compatible poly(e-caprolactone)-block-poly(l-lactic acid) copolymer film surfaces. Colloids Surf. B Biointerfaces 2014, 114, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, A.; Choi, W.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Robust Omniphobic Surfaces. Proc. Natl. Acad. Sci. USA 2008, 105, 18200–18205. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired Surfaces with Special Wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, M.; Jiang, L. Recent developments in polymeric superoleophobic surfaces. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1209–1224. [Google Scholar] [CrossRef]
- Maani, N.; Rayz, V.S.; Nosonovsky, M. Biomimetic approaches for green tribology: From the lotus effect to blood flow control. Metrol. Prop 2015, 3, 034001. [Google Scholar] [CrossRef]
- Cheng, D.F.; Masheder, B.; Urata, C.; Hozumi, A. Smooth perfluorinated surfaces with different chemical and physical natures: Their unusual dynamic dewetting behavior toward polar and nonpolar liquids. Langmuir 2013, 29, 11322–11329. [Google Scholar] [CrossRef]
- Wu, X.; Liu, M.; Zhong, X.; Liu, G.; Wyman, I.; Wang, Z.; Wu, Y.; Yang, H.; Wang, J. Smooth Water-Based Antismudge Coatings for Various Substrates. Sustain. Chem. Eng. 2017, 5, 2605–2613. [Google Scholar] [CrossRef]
- Cheng, D.F.; Urata, C.; Masheder, B.; Hozumi, A. A physical approach to specifically improve the mobility of alkane liquid drops. J. Am. Chem. Soc. 2012, 134, 10191–10199. [Google Scholar] [CrossRef]
- Urata, C.; Masheder, B.; Cheng, D.F.; Hozumi, A. How to reduce resistance to movement of alkane liquid drops across tilted surfaces without relying on surface roughening and perfluorination. Langmuir 2012, 28, 17681–17689. [Google Scholar] [CrossRef] [PubMed]
- Urata, C.; Cheng, D.F.; Masheder, B.; Hozumi, A. Smooth, transparent and nonperfluorinated surfaces exhibiting unusual contact angle behavior toward organic liquids. RSC Adv. 2012, 2, 9805–9808. [Google Scholar] [CrossRef]
- Urata, C.; Masheder, B.; Cheng, D.F.; Hozumi, A. Unusual dynamic dewetting behavior of smooth perfluorinated hybrid films: Potential advantages over conventional textured and liquid-infused perfluorinated surfaces. Langmuir 2013, 29, 12472–12482. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, H.; He, W.; Li, H.; Jiang, J.; Liu, M.; Sun, H.; He, M.; Cui, J.; Jiang, L.; et al. Development of “liquid-like” Copolymer Nanocoatings for Reactive Oil-Repellent Surface. ACS Nano 2017, 11, 2248–2256. [Google Scholar] [CrossRef]
- Khan, F.; Rabnawaz, M.; Li, Z.; Khan, A.; Naveed, M.; Tuhin, M.O.; Rahimb, F. Simple Design for Durable and Clear Self-Cleaning Coatings. ACS Appl. Polym. Mater. 2019, 1, 2659–2667. [Google Scholar] [CrossRef]
- Zhong, X.; Lv, L.; Hu, H.; Jiang, X.; Fu, H. Bio-based coatings with liquid repellency for various applications. Chem. Eng. J. 2020, 382, 123042. [Google Scholar] [CrossRef]
- Wang, L.; McCarthy, T.J. Covalently Attached Liquids: Instant Omniphobic Surfaces with Unprecedented Repellency. Angew. Chem. Int. Ed. 2016, 55, 244–248. [Google Scholar] [CrossRef]
- Cheng, D.F.; Urata, C.; Yagihashi, M.; Hozumi, A. A statically oleophilic but dynamically oleophobic smooth nonperfluorinated surface. Angew. Chem. Int. Ed. 2012, 51, 2956–2959. [Google Scholar] [CrossRef]
- Fadeev, A.Y.; Mccarthy, T.J. Trialkylsilane Monolayers Covalently Attached to Silicon Surfaces: Wettability Studies Indicating that Molecular Topography Contributes to Contact Angle Hysteresis. Langmuir 1999, 15, 3759–3766. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, X.; Lu, C.; Bai, L.; Guan, H.; Tong, Y. A facile approach to fabricate dynamically omniphobic coating on diverse substrates for self-cleaning. Prog. Org. Coat. 2019, 132, 475–480. [Google Scholar] [CrossRef]
- Krumpfer, J.W.; McCarthy, T.J. Rediscovering silicones: “unreactive” silicones react with inorganic surfaces. Langmuir 2011, 27, 11514–11519. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.E. Some interesting things about polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Longenberger, T.B.; Ryan, K.M.; Bender, W.Y.; Krumpfer, A.-K.; Krumpfer, J.W. The Art of Silicones: Bringing Siloxane Chemistry to the Undergraduate Curriculum. J. Chem. Educ. 2017, 94, 1682–1690. [Google Scholar] [CrossRef]
- Smallwood, I.M. Handbook of Organic Solvent Properties; Wiley: London, UK; Halsted Press: New York, NY, USA, 1996. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2013; Volume 53, ISBN 9788578110796. [Google Scholar]
- Korosi, G.; Kovats, E.S. Density and surface tension of 83 organic liquids. J. Chem. Eng. Data 1981, 26, 323–332. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003; Volume 53, ISBN 0849304849. [Google Scholar]
- Mittal, K.L. Silanes and Other Coupling Agents; LEIDEN: Boston, MA, USA, 2007. [Google Scholar]
- Mark, J.E. Polymer Data Handbook, 2nd ed.; Oxford University Press: New York, NY, USA, 1999; ISBN 9780195107890. [Google Scholar]
- Papazian, H.A. Correlation of Surface Tension between Various Liquids. J. Am. Chem. Soc. 1971, 93, 5634–5636. [Google Scholar] [CrossRef]
- Koenhen, D.M. The determination of solubility parameters of solvents and polymers by means of correlations with other physical quantities. J. Appl. Polym. Sci. 1975, 19, 1163–1179. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Shi, B. A new equation between surface tensions and solubility parameters without molar volume parameters simultaneously fitting polymers and solvents. J. Macromol. Sci. Part B Phys. 2011, 50, 1042–1046. [Google Scholar] [CrossRef]










| Probe Liquid | Dielectric Constant | Surface Tension (mN/m) | Solubility Parameter (calcm−3)0.5 | Viscosity (mPaS) |
|---|---|---|---|---|
| Water a | 79.7 | 72.8 | 23.4 | 0.89 |
| Acetonitrile a | 37.5 | 29.1 | 11.9 | 0.38 |
| DMF a | 36.7 | 35.0 | 12.1 | 0.82 |
| Ethanol a | 22.4 | 22.3 | 12.7 | 1.08 |
| Diiodomethane | 5.3 d | 50.8 c | 9.3 e | 2.76 |
| Toluene a | 2.4 | 28.5 | 8.9 | 0.59 |
| 1,4-Dioxane a | 2.2 | 33.3 c | 10.0 | 1.30 |
| n-hexadecane | 2.1 d | 27.5 c | 8.0 b | 3.08 d |
| 1-Propanol a | 20.1 | 23.7 | 11.9 | 1.72 |
| 1-Butanol a | 18.2 | 24.6 | 11.4 | 3.0 |
| 1-Octanol a | 10.3 d | 27.5 | 10.4 | 7.5 |
| DMSO a | 46.6 | 43.7 | 13.0 | 2.0 |
| PDMS f | 2.5 | 20.8 | 7.5 | − |
| Ra (nm) | |
|---|---|
| Dry | |
| Water | |
| n-hexadecane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monder, H.; Bielenki, L.; Dodiuk, H.; Dotan, A.; Kenig, S. Poly (Dimethylsiloxane) Coating for Repellency of Polar and Non-Polar Liquids. Polymers 2020, 12, 2423. https://doi.org/10.3390/polym12102423
Monder H, Bielenki L, Dodiuk H, Dotan A, Kenig S. Poly (Dimethylsiloxane) Coating for Repellency of Polar and Non-Polar Liquids. Polymers. 2020; 12(10):2423. https://doi.org/10.3390/polym12102423
Chicago/Turabian StyleMonder, Hila, Leo Bielenki, Hanna Dodiuk, Anna Dotan, and Samuel Kenig. 2020. "Poly (Dimethylsiloxane) Coating for Repellency of Polar and Non-Polar Liquids" Polymers 12, no. 10: 2423. https://doi.org/10.3390/polym12102423