Barrier Film of Etherified Hemicellulose from Single-Step Synthesis
Abstract
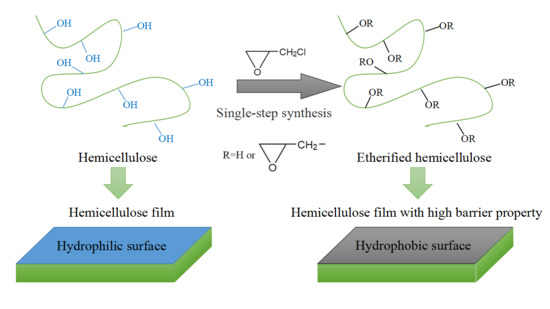
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction and Etherification of Poplar Hemicellulose
2.3. Preparation of Etherified Hemicellulose Films
2.4. Analytical Methods
3. Results and Discussion
3.1. Structural Analysis of Etherified Hemicellulose Films
3.2. Mechanical Properties of Modified Hemicellulose Films
3.3. Thermal Stability Analysis of Modified Hemicellulose Films
3.4. Surface Hydrophobicity Analysis of Modified Hemicellulose Films
3.5. Oxygen Resistance Performance of Modified Hemicellulose Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, G.-G.; Qi, X.-M.; Guan, Y.; Peng, F.; Yao, C.-L.; Sun, R.-C. High Strength Hemicellulose-Based Nanocomposite Film for Food Packaging Applications. ACS Sustain. Chem. Eng. 2016, 4, 1985–1993. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Chang, C.; Wang, Y. Recent developments and prospective food-related applications of cellulose nanocrystals: A review. Cellulose 2020, 27, 2991–3011. [Google Scholar] [CrossRef]
- Huang, S.; Tao, R.; Ismail, A.; Wang, Y. Cellulose Nanocrystals Derived from Textile Waste through Acid Hydrolysis and Oxidation as Reinforcing Agent of Soy Protein Film. Polymers 2020, 12, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Y.; Ren, J.L.; Li, W.Y.; Sun, R.C.; Liu, S.J. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Gordobil, O.; Egues, I.; Urruzola, I.; Labidi, J. Xylan-cellulose films: Improvement of hydrophobicity, thermal and mechanical properties. Carbohydr. Polym. 2014, 112, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.H.; Waldron, K.W.; Wilson, D.R.; Cunha, A.P.; De Brito, E.S.; Rodrigues, T.H.; Rosa, M.F.; De Azeredo, H.M.C. Wheat straw hemicelluloses added with cellulose nanocrystals and citric acid. Effect on film physical properties. Carbohydr. Polym. 2017, 164, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, E.; Guo, J.Q.; Yang, F.; Zhu, Y.Y.; Song, J.L.; Jin, Y.C.; Rojas, O.J. On the polymorphic and morphological changes of cellulose nanocrystals (CNC-I) upon mercerization and conversion to CNC-II. Carbohydr. Polym. 2016, 143, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.B.; Wang, J.; He, Y.; Sun, H.; Chen, X.L.; Zheng, Q.; Xie, H.B. Triply Biobased Thermoplastic Composites of Polylactide/Succinylated Lignin/Epoxidized Soybean Oil. Polymers 2020, 12, 632. [Google Scholar] [CrossRef] [Green Version]
- Mendes, F.R.S.; Bastos, M.S.R.; Mendes, L.G.; Silva, A.R.A.; Sousa, F.D.; Monteiro-Moreira, A.C.O.; Cheng, H.N.; Biswas, A.; Moreira, R.A. Preparation and evaluation of hemicellulose films and their blends. Food Hydrocoll. 2017, 70, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Hu, Y.; Yang, B.; Xu, G.Z. Valorization of Agricultural and Forestry Biomass Byproducts: Progress in Isolation, Modification and Application of Hemicellulose. China Plast. 2016, 30, 12–22. [Google Scholar]
- Huang, J.Z.; Liu, Y.X.; Sun, B.; Li, J.Y.; Zhang, R.F.; Nie, S.X. Laccase Pretreatment for Enhancing Microwave-assisted Alkaline Extraction of Hemicellulose from Bagasse. Bioresources 2019, 14, 931–942. [Google Scholar] [CrossRef]
- Braga, R.D.; Poletto, M. Preparation and Characterization of Hemicellulose Films from Sugarcane Bagasse. Materials 2020, 13, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.X.; Sun, B.; Wang, Z.L.; Ni, Y.H. Mechanical and Water Vapor Barrier Properties of Bagasse Hemicellulose-based Films. Bioresources 2016, 11, 4226–4236. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, N.; Chen, M.; Wei, Y.; Liu, C. Functional packaging films originating from hemicelluloses laurate by direct transesterification in ionic liquid. Carbohydr. Polym. 2019, 229, 115336. [Google Scholar] [CrossRef]
- Hansen, N.M.L.; Plackett, D. Sustainable Films and Coatings from Hemicelluloses: A Review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Heikkilä, M.I.; Willför, S.M.; Tenkanen, M.; Mikkonen, K.S.; Heikkilä, M.I.; Willför, S.M.; Tenkanen, M. Films from glyoxal-crosslinked spruce galactoglucomannans plasticized with sorbitol. Int. J. Polym. Sci. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Stepan, A.M.; Ansari, F.; Berglund, L.; Gatenholm, P. Nanofibrillated cellulose reinforced acetylated arabinoxylan films. Compos. Sci. Technol. 2014, 98, 72–78. [Google Scholar] [CrossRef]
- Chen, G.-G.; Qi, X.-M.; Li, M.-P.; Guan, Y.; Bian, J.; Peng, F.; Yao, C.-L.; Sun, R.-C. Hemicelluloses/montmorillonite hybrid films with improved mechanical and barrier properties. Sci. Rep. 2015, 5, 16405. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef] [Green Version]
- Nypelö, T.; Laine, C.; Aoki, M.; Tammelin, T.; Henniges, U. Etherification of Wood-Based Hemicelluloses for Interfacial Activity. Biomacromolecules 2016, 17, 1894–1901. [Google Scholar] [CrossRef]
- Peresin, M.S.; Kammiovirta, K.; Setälä, H.; Tammelin, T. Structural Features and Water Interactions of Etherified Xylan Thin Films. J. Polym. Env. 2012, 20, 895–904. [Google Scholar] [CrossRef]
- Hettrich, K.; Drechsler, U.; Loth, F.; Volkert, B. Preparation and Characterization of Water-Soluble Xylan Ethers. Polymers 2017, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Ren, J.-L.; Sun, R.-C. Homogeneous Esterification of Xylan-Rich Hemicelluloses with Maleic Anhydride in Ionic Liquid. Biomacromolecules 2010, 11, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Gröndahl, M.; Gustafsson, A.; Gatenholm, P. Gas-Phase Surface Fluorination of Arabinoxylan Films. Macromolecules 2006, 39, 2718–2721. [Google Scholar] [CrossRef]
- Guan, Y.; Qi, X.M.; Zhang, B.; Chen, G.G.; Peng, F.; Sun, R.C. Physically Crosslinked Composite Hydrogels of Hemicelluloses with Poly (vinyl alcohol phosphate) and Chitin Nanowhiskers. Bioresources 2015, 10, 1378–1393. [Google Scholar] [CrossRef]
- Du, J.; Li, C.; Zhao, Y.; Wang, H. Hemicellulose isolated from waste liquor of viscose fiber mill for preparation of polyacrylamide-hemicellulose hybrid films. Int. J. Boil. Macromol. 2018, 108, 1255–1260. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, G. Branching of hemicelluloses through an azetidinium salt ring-opening reaction. Carbohydr. Res. 2016, 428, 23–30. [Google Scholar] [CrossRef]
- Kong, W.Q.; Ren, J.L.; Wang, S.Y.; Li, M.F.; Sun, R.C. A Promising Strategy for Preparation of Cationic Xylan by Environment-Friendly Semi-Dry Oven Process. Fibers Polym. 2014, 15, 943–949. [Google Scholar] [CrossRef]
- Shao, H.; Sun, H.; Yang, B.; Zhang, H.J.; Hu, Y. Facile and green preparation of hemicellulose-based film with elevated hydrophobicity via cross-linking with citric acid. RSC Adv. 2019, 9, 2395–2401. [Google Scholar] [CrossRef] [Green Version]
- Farhat, W.; Venditti, R.; Ayoub, A.; Prochazka, F.; Fernandez-De-Alba, C.; Mignard, N.; Taha, M.; Becquart, F. Towards thermoplastic hemicellulose: Chemistry and characteristics of poly-(ε-caprolactone) grafting onto hemicellulose backbones. Mater. Des. 2018, 153, 298–307. [Google Scholar] [CrossRef]
- Huang, C.; Fang, G.; Deng, Y.; Bhagia, S.; Meng, X.; Tao, Y.; Yong, Q.; Ragauskas, A.J. Robust galactomannan/graphene oxide film with ultra-flexible, gas barrier and self-clean properties. Compos. Part A Appl. Sci. Manuf. 2020, 131, 105780. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Sun, H.; Yang, B.; Weng, Y.X. Hemicellulose-Based Film: Potential Green Films for Food Packaging. Polymers 2020, 12, 1775. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhou, Y.K.; Waterhouse, G.I.N.; Gong, R.Z.; Xie, J.Z.; Zhang, K.; Xu, J. Optimizing interfacial adhesion in PBAT/PLA nanocomposite for biodegradable packaging films. Food Chem. 2020, 334, 127487. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Takahashi, H.; Noguchi, A. Improved water resistance in edible zein films and composites for biodegradable food packaging. Int. J. Food Sci. Technol. 1995, 30, 599–608. [Google Scholar] [CrossRef]
- Philo, M.R.; Damant, A.P.; Castle, L. Reactions of epoxide monomers in food simulants used to test plastics for migration. Food Addit. Contam. 1997, 14, 75–82. [Google Scholar] [CrossRef]
- Härdelin, L.; Bernin, D.; Börjesson, M.; Strom, A.; Larsson, A. Altered Thermal and Mechanical Properties of Spruce Galactoglucomannan Films Modified with an Etherification Reaction. Biomacromolecules 2020, 21, 1832–1840. [Google Scholar] [CrossRef]
- Ibn Yaich, A.; Edlund, U.M.; Albertsson, A.-C. Transfer of Biomatrix/Wood Cell Interactions to Hemicellulose-Based Materials to Control Water Interaction. Chem. Rev. 2017, 117, 8177–8207. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Jiang, L. Contact angle measurement of natural materials. Colloids Surf. B Biointerfaces 2018, 161, 324–330. [Google Scholar] [CrossRef]
- Cwiklik, L.; Jagoda-Cwiklik, B.; Frankowicz, M. Distribution geometry of active centers and efficiency of heterogeneous reaction. In Proceedings of the 3rd European Interdisciplinary School on Nonlinear Dynamics for System and Signal Analysis, Warsaw, Poland, 18–27 June 2002; pp. 295–300. [Google Scholar]
- Xue, Y.Q.; Cui, Z.X. Study on basic-catalyzed hydrolysis of epichlorohydrin. Appl. Chem. Ind. 2010, 39, 49–51. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.Y.; Yang, Q.; Lu, D.N. Bacterial cellulose & poly (vinyl alcohol) nanocomposite hydrogels prepared by chemical crosslinking. J. Appl. Polym. Sci. 2012, 126, E245–E251. [Google Scholar] [CrossRef]
- Hartman, J.; Albertsson, A.C.; Sjöberg, J. Surface- and Bulk-Modified Galactoglucomannan Hemicellulose Films and Film Laminates for Versatile Oxygen Barriers. Biomacromolecules 2006, 7, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, K.S.; Heikkilä, M.I.; Helén, H.; Hyvönen, L.; Tenkanen, M. Spruce galactoglucomannan films show promising barrier properties. Carbohydr. Polym. 2010, 79, 1107–1112. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Heikkinen, S.; Soovre, A.; Peura, M.; Serimaa, R.; Talja, R.A.; Helen, H.; Hyvonen, L.; Tenkanen, M. Films from Oat Spelt Arabinoxylan Plasticized with Glycerol and Sorbitol. J. Appl. Polym. Sci. 2009, 114, 457–466. [Google Scholar] [CrossRef]
- Kisonen, V.; Prakobna, K.; Xu, C.L.; Salminen, A.; Mikkonen, K.S.; Valtakari, D.; Eklund, P.; Seppala, J.; Tenkanen, M.; Willfor, S. Composite films of nanofibrillated cellulose and O-acetyl galactoglucomannan (GGM) coated with succinic esters of GGM showing potential as barrier material in food packaging. J. Mater. Sci. 2015, 50, 3189–3199. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Laine, C.; Kontro, I.; Talja, R.A.; Serimaa, R.; Tenkanen, M. Combination of internal and external plasticization of hydroxypropylated birch xylan tailors the properties of sustainable barrier films. Eur. Polym. J. 2015, 66, 307–318. [Google Scholar] [CrossRef]
- Weber, C.J. Biobased Packaging Materials for the Food Industry–Status and Perspectives; Weber, C.J., Ed.; KVL Department of Dairy and Food Science: Frederiksberg, Denmark, 2000. [Google Scholar]










| Sample | Dosage of Epoxy Chloropropane (mL) | Feed Ratio of Epoxy Chloropropane (mL/g) | Mass of Hemicellulose (g) | Mass of PVA (g) | Mass of Sorbitol (g) |
|---|---|---|---|---|---|
| E0 | 0 | 0 | 0.90 | 0.30 | 0.30 |
| E5 | 5 | 1/12 | 0.90 | 0.30 | 0.30 |
| E10 | 10 | 1/6 | 0.90 | 0.30 | 0.30 |
| E20 | 20 | 1/3 | 0.90 | 0.30 | 0.30 |
| E40 | 40 | 2/3 | 0.90 | 0.30 | 0.30 |
| E60 | 60 | 1/1 | 0.90 | 0.30 | 0.30 |
| E80 | 80 | 4/3 | 0.90 | 0.30 | 0.30 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| E0 | 7.44 ± 0.44 | 4.36 ± 0.06 | E40 | 14.60 ± 1.33 | 2.53 ± 0.22 |
| E5 | 8.60 ± 0.59 | 5.65 ± 1.19 | E60 | 10.68 ± 0.37 | 5.05 ± 0.65 |
| E10 | 10.07 ± 0.83 | 5.53 ± 0.84 | E80 | 7.62 ± 0.66 | 6.12 ± 0.52 |
| E20 | 11.46 ± 1.90 | 5.48 ± 1.32 |
| Sample | Oxygen Permeability [(cm3·µm)/(m2·d·kPa)] | Reference Citation |
|---|---|---|
| E0 | 1053 1 | - |
| E5 | 204 1 | - |
| E10 | 26.5 1 | - |
| E20 | 4.3 1 | - |
| E40 | 1.9 1 | - |
| E60 | 5.2 1 | - |
| E80 | 18.6 1 | - |
| Spruce galactoglucomannan | 6.8 | [43] |
| Oat spelt arabinoxylan with 40% sorbitol | 4.7 | [44] |
| O-acetyl-galactoglucomannan with nanofibrillated cellulose | 3.2 | [45] |
| Hydroxypropylated birch xylan | 4.7–24 | [46] |
| Quaternized hemicelluloses with chitosan and montmorillonite | 10.95–16.37 | [1] |
| Low-density polyethylene (LDPE) | 7900 | [47] |
| Poly(lactic acid) (PLA) | 160 | [47] |
| Poly(hydroxyalkanoate) (PHA) | 150 | [47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, H.; Zhao, Y.; Sun, H.; Yang, B.; Fan, B.; Zhang, H.; Weng, Y. Barrier Film of Etherified Hemicellulose from Single-Step Synthesis. Polymers 2020, 12, 2199. https://doi.org/10.3390/polym12102199
Shao H, Zhao Y, Sun H, Yang B, Fan B, Zhang H, Weng Y. Barrier Film of Etherified Hemicellulose from Single-Step Synthesis. Polymers. 2020; 12(10):2199. https://doi.org/10.3390/polym12102199
Chicago/Turabian StyleShao, Hui, Yuelong Zhao, Hui Sun, Biao Yang, Baomin Fan, Huijuan Zhang, and Yunxuan Weng. 2020. "Barrier Film of Etherified Hemicellulose from Single-Step Synthesis" Polymers 12, no. 10: 2199. https://doi.org/10.3390/polym12102199