Fabrication of Hybrid Membranes Containing Nylon-11 and Organic Semiconductor Particles with Potential Applications in Molecular Electronics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of π-Conjugated Molecular Semiconductors
2.2. Membrane Fabrication and Characterization
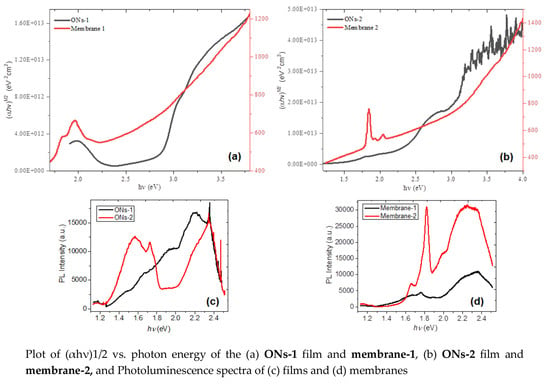
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zimmerman, J.; Parameswaran, R.; Tian, B. Nanoscale Semiconductor Devices as New Biomaterials. Biomater. Sci. 2014, 5, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Xu, S.; Rogers, J.; Cestellos-Blanco, S. Roadmap on semiconductor–cell biointerfaces. Phys. Biol. 2018, 15, 2–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timko, B.; Cohen-Karni, T.; Qing, Q. Design and Implementation of Functional Nanoelectronic Interfaces with Biomolecules, Cells, and Tissue using Nanowire Device Array. IEEE Trans. Nanotechnol. 2010, 9, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.; Lieber, C. Design, synthesis, and characterization of novel nanowire structures for photovoltaics and intracellular probe. Pure Appl. Chem. 2011, 83, 2153–2169. [Google Scholar] [CrossRef] [Green Version]
- Rak, J.; Pouckova, P.; Benes, J.; Vetvicka, D. Drug Delivery Systems for Phthalocyanines for Photodynamic Therapy. Anticancer Res. 2019, 39, 3323–3339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paula, L.B.; Primo, F.L.; Pinto, M.R.; Morais, P.C.; Tedesco, A.C. Evaluation of a chloroaluminium phthalocyanine-loaded magnetic nanoemulsion as a drug delivery device to treat glioblastoma using hyperthermia and photodynamic therapy. RSC Adv. 2017, 7, 9115–9122. [Google Scholar] [CrossRef] [Green Version]
- Kladsomboon, S.; Thippakorn, C.; Seesaard, T. Development of Organic-Inorganic Hybrid Optical Gas Sensors for the Non-Invasive Monitoring of Pathogenic Bacteria. Sensors 2018, 18, 3189. [Google Scholar] [CrossRef] [Green Version]
- Adiga, S.; Jin, C.; Curtiss, L.; Monteiro-Riviere, N.; Narayan, R. Nanoporous membranes for medical and biological applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 568–581. [Google Scholar] [CrossRef] [Green Version]
- Hamui, L.; Sánchez-Vergara, M.E.; Sánchez-Ruiz, R.; Ruanova-Ferreiro, D.; Ballinas Indili, R.; Álvarez-Toledano, C. New Development of Membrane Base Optoelectronic Devices. Polymers 2018, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Wang, H.; Yao, X.; Bi, D.; Zhu, G.; Tang, S.; Wei, J.; Yang, L.; Tong, P.; Xiao, L. Development of nanofluorapatite polymer-based composite for bioactive orthopedic implants and prostheses. Int. J. Nanomed. 2014, 9, 3875–3884. [Google Scholar] [CrossRef] [Green Version]
- Bugatti, V.; Vertuccio, L.; Viscusi, G.; Gorrasi, G. Antimicrobial Membranes of Bio-Based PA 11 and HNTs Filled with Lysozyme Obtained by an Electrospinning Process. Nanomaterials 2018, 8, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mark, J.E. Polymer Data Handbook; Oxford University Press, Inc.: New York, NY, USA, 1999; p. 221. [Google Scholar]
- Barzin, J.; Feng, C.; Khulbe, K.C.; Matsuura, T.; Madaeni, S.S.; Mirzadeh, H. Characterization of polyethersulfone hemodialysis membrane by ultrafiltration and atomic force microscopy. J. Membr. Sci. 2004, 237, 77–85. [Google Scholar] [CrossRef]
- Schowalter, S.J.; Connolly, C.B.; Doyle, J.M. Permeability of noble gases through Kapton, butyl, nylon, and “Silver Shield”. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 2010, 615, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Habib, B.; Elham, A.; Ahmad, B. Permeability of methane, carbon dioxide, oxygen and nitrogen gases using a composite membrane of polyethersulfone/polyamide 11. Int. J. Adv. Biotechnol. Res. 2016, 7, 965–974. Available online: https://bipublication.com/files/ijabr201605118_Ameri.pdf (accessed on 10 December 2019).
- Flaconnèche, B.; Martin, J.; Klopffer, M.H. Permeability, Diffusion and Solubility of Gases in Polyethylene, Polyamide 11 and Poly (Vinylidene Fluoride). Oil Gas Sci. Technol. 2001, 56, 261–278. [Google Scholar] [CrossRef] [Green Version]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural Characterization of Thin-Film Polyamide Reverse Osmosis Membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Mafukidze, D.M.; Mashazi, P.; Nyokong, T. Synthesis and singlet oxygen production by a phthalocyanine when embedded in asymmetric polymer membranes. Polymer 2016, 105, 203–213. [Google Scholar] [CrossRef]
- Francesco, R.; Vanna, T. Metal-Polymer Nanocomposites: (Co-) Evaporation/(Co) Sputtering Approaches and Electrical Properties. Coatings 2015, 5, 378–424. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, M.; Pesce, L.; Pezzuoli, D.; Montali, C.; Brancaleon, L.; Cavanna, L.; Abbruzzetti, S.; Diaspro, A.; Bianchini, P.; Viappiani, C. Apomyoglobin is an efficient carrier for zinc phthalocyanine in photodynamic therapy of tumors. Biophys. Chem. 2019, 253, 106228. [Google Scholar] [CrossRef]
- Dodabalapur, A.; Katz, H.E.; Torsi, L.; Haddon, R.C. Organic Heterostructure Field-Effect Transistors. Science 1995, 269, 1560–1562. [Google Scholar] [CrossRef] [PubMed]
- Kayunkid, N.; Rangkasikorn, A.; Saributr, C.; Nukeaw, J. Growth and characterizations of tin doped zinc-phthalocyanine prepared by thermal co-evaporation in high vacuum as a nanomaterial. Jpn. J. Appl. Phys. 2016, 55, 02BB12. [Google Scholar] [CrossRef]
- Li, B.; Noda, Y.; Hu, L.; Yoshikawa, H.; Matsushita, M.M.; Awaga, K. Electric double layers allow for opaque electrodes in high performance organic optoelectronic devices. Appl. Phys. Lett. 2012, 100, 163304. [Google Scholar] [CrossRef]
- Pfuetzner, S.; Meiss, J.; Petrich, A.; Riede, M.; Leo, K. Improved bulk heterojunction organic solar cells employing C70 fullerenes. Appl. Phys. Lett. 2009, 94, 253303. [Google Scholar] [CrossRef]
- Zugle, R.; Antunes, E.; Khene, S.; Nyokong, T. Photooxidation of 4-chlorophenol sensitized by lutetium tetraphenoxy phthalocyanine anchored on electrospun polystyrene polymer fiber. Polyhedron 2012, 33, 74–81. [Google Scholar] [CrossRef]
- Zugle, R.; Nyokong, T. Zinc (II) 2,9,16,23-tetrakis[4-(N-methylpyridyloxy)]-phthalocyanine anchored on an electrospun polysulfone polymer fiber: Application for photosensitized conversion of methyl orange. J. Mol. Catal. A Chem. 2013, 366, 247–253. [Google Scholar] [CrossRef]
- Zugle, R.; Litwinski, C.; Torto, N.; Nyokong, T. Photophysical and photochemical behavior of electrospun fibers of a polyurethane polymer chemically linked to lutetium carboxyphenoxy phthalocyanine. New J. Chem. 2011, 35, 1588–1595. [Google Scholar] [CrossRef]
- Modisha, P.; Nyokong, T. Fabrication of phthalocyanine-magnetic nanoparticles hybrid nanofibers for degradation of Orange-G. J. Mol. Catal. A Chem. 2014, 381, 132–137. [Google Scholar] [CrossRef]
- Khoza, P.; Nyokong, T. Visible light transformation of Rhodamine 6G using tetracarbazole zinc phthalocyanine when embedded in electrospun fibers and in the presence of ZnO and Ag particles. J. Coord. Chem. 2015, 68, 1117–1131. [Google Scholar] [CrossRef]
- Goethals, A.; Mugadza, T.; Arslanoglu, Y.; Zugle, R.; Antunes, E.; Van Hulle, S.W.H.; Nyokong, T.; De Clerck, K. Polyamide nanofiber membranes functionalized with zinc phthalocyanines. J. Appl. Polym. Sci. 2014, 131, 13. [Google Scholar] [CrossRef] [Green Version]
- Łapok, L.; Cyza, M.; Gut, A.; Kępczyński, M.; Szewczyk, G.; Sarna, T.; Nowakowska, M. Synthesis, spectroscopic properties and interaction with a liposomal membrane of a novel iodinated magnesium phthalocyanine. J. Photochem. Photobiol. A Chem. 2014, 286, 55–63. [Google Scholar] [CrossRef]
- Pashkovskaya, A.A.; Perevoshchikova, I.V.; Maizlish, V.E.; Shaposhnikov, G.P.; Kotova, E.A.; Antonenko, Y.N. Interaction of Tetrasubstituted Cationic Aluminum Phthalocyanine with Artificial and Natural Membranes. Biochemistry 2009, 74, 9, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.N.; Henkensmeier, D.; Park, Y.-H.; Jang, J.-H.; Kwon, T.; Koo, C.H.; Kim, H.-J.; Han, J.; Nam, S.W. Blue membranes: Sulfonated copper(II) phthalocyanine tetrasulfonic acid based composite membranes for DMFC and low relative humidity PEMFC. J. Membr. Sci. 2016, 502, 1–10. [Google Scholar] [CrossRef]
- Preethi, N.; Shinohara, H.; Nishide, H. Reversible oxygen-binding and facilitated oxygen transport in membranes of polyvinylimidazole complexed with cobalt-phthalocyanine. React. Funct. Polym. 2006, 66, 851–855. [Google Scholar] [CrossRef]
- Heeger, A.J. 25th Anniversary Article: Bulk Heterojunction Solar Cells: Understanding the Mechanism of Operation. Adv. Mat. 2014, 26, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Luciana, G.; Cordeiro, M.R.; Freitas, A.R.; Moreira, W.C.; Girotto, E.M.; Zucolotto, V. The effects of temperature on the molecular orientation of zinc phthalocyanine films. J. Mater. Sci. 2010, 45, 1366–1370. [Google Scholar] [CrossRef]
- Seoudi, R.; El-Bahy, G.S.; El Sayed, Z.A. FTIR, TGA and DC electrical conductivity studies of phthalocyanine and its complexes. J. Mol. Struct. 2005, 753, 119–126. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N.; Rasga, O.; Breazua, C.; Stavarache, I.; Stanculescu, F.; Socol, G.; Gherendi, F.; Grumezescu, V.; Popescu-Pelin, G.; et al. Flexible heterostructures based on metal phthalocyanines thin filmsobtained by MAPLE. Appl. Surf. Sci. 2016, 374, 403–410. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Farag, A.M.; El-Rahman, A.b.d.; Darwish, A.A.A. Dispersion Studies and Electronic Transitions in Nickel Phthalocyanine Thin Films. Opt. Laser Technol. 2005, 37, 513–523. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Abd-El-Rahman, K.F.; Al-Ghamdi, A.A.; Asiri, A.M. Optical properties of thermally evaporated tin-phthalocyanine dichloride thin films, SnPcCl2. Phys. B Condens. Matter 2004, 344, 398–406. [Google Scholar] [CrossRef]
- Melby, L.R.; Hartzler, H.D.; Sheppard, W.A. An Improved Synthesis of tetrathiafulvalene. J. Org. Chem. 1974, 39, 2456–2458. [Google Scholar] [CrossRef]
- Rand, B.P.; Cheyns, D.; Vasseur, K.; Giebink, N.C.; Mothy, S.; Coropceanu, Y.; Yi, V.; Beljonne, D.; Cornil, J.; Brédas, J.L.; et al. The Impact of Molecular Orientation on the Photovoltaic Properties of a Phthalocyanine/Fullerene Heterojunction. Adv. Funct. Mater. 2012, 22, 2987–2995. [Google Scholar] [CrossRef]
- Della Pirriera, M.; Puigdollers, J.; Voz, C.; Stella, M.; Bertomeu, J.; Alcubilla, R. Optoelectronic properties of CuPc thin films deposited at different substrate temperatures. J. Phys. D Appl. Phys. 2009, 42, 145102. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, S. Spin-Dependent Processes in Organic Devices. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2010. [Google Scholar]
- Saleh, A.M.; Hassan, A.K.; Gould, R.D. DC conduction processes and electrical parameters of the organic semiconducting zinc phthalocyanine, ZnPc, thin films. J. Phys. Chem. Solids 2003, 64, 1297–1303. [Google Scholar] [CrossRef]
- Zanfolim, A.A.; Volpati, D.; Olivati, C.A.; Job, A.E.; Constantino, C.J.L. Structural and Electric-Optical Properties of Zinc Phthalocyanine Evaporated Thin Films: Temperature and Thickness Effects. J. Phys. Chem. 2010, 114, 12290–12299. [Google Scholar] [CrossRef]
- Saleh, A.M.; Abu-Hilal, A.O.; Gould, R.D. Investigation of electrical properties (ac and dc) of organic zinc phthalocyanine, ZnPc, semiconductor thin films. Curr. Appl. Phys. 2003, 3, 345–350. [Google Scholar] [CrossRef]
- Gao, W.; Khan, A. Electronic structure and current injection in zinc phthalocyanine doped with tetrafluorotetracyanoquinodimethane: Interface versus bulk effects. Org. Electron. 2002, 3, 53–63. [Google Scholar] [CrossRef]
- Gao, W.; Khan, A. Controlled p-doping of zinc phthalocyanine by coevaporation with tetrafluorotetracyanoquinodimethane: A direct and inverse photoemission study. Appl. Phys. Lett. 2001, 79, 4040–4042. [Google Scholar] [CrossRef] [Green Version]
- Gould, R.D. Structure and electrical conduction properties of phthalocyanine thin films. Coord. Chem. Rev. 1996, 156, 237–274. [Google Scholar] [CrossRef]
- Bisquert, J.; Montero, J.M.; Bolink, H.J.; Barea, E.M.; Belmonte, G. Thickness scaling of space-charge-limited currents in organic layers with field -or density- dependent mobility. Phys. Stat. Sol. 2006, 15, 3762–3767. [Google Scholar] [CrossRef]
- Anthopoulos, T.D.; Shafai, T.S. SCLC Measurements in Nickel Phthalocyanine Thin Films. Phys. Stat. Sol. 2000, 181, 569–574. [Google Scholar] [CrossRef]
- Gravano, S.; Hassan, A.K.; Gould, R.D. Effects of annealing on the trap distribution of cobalt phthalocyanine thin films. Int. J. Electron. 1991, 70, 477–484. [Google Scholar] [CrossRef]
- Hassan, A.K.; Gould, R.D. The interpretation of current density-voltage and activation energy measurements on freshly prepared and heat-treated nickel phthalocyanine thin films. Int. J. Electron. 1993, 74, 59–65. [Google Scholar] [CrossRef]
- Toader, M.; Gopakumar, T.G.; Abdel-Hafiez, M.; Hietshould, M. Exploring the F16CoPc/Ag(110) Interface Using Scanning Tunneling Microscopy and Spectroscopy. Part 1: Template-Guided Adlayer Structure Formation. J. Phys. Chem. C 2010, 114, 3537–3543. [Google Scholar] [CrossRef]
- Urbach, F. The Long-Wavelength Edge of Photographic Sensitivity and of the Electronic Absorption of Solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- García-Moreno, G. Pi-Conjugated Organic Semiconductor Based on Thiophene. A Theorical Study. Ph.D. Thesis, University of Jaén Faculty of Experimental Science, Jaén, Spain, 2012. [Google Scholar]
- González, S.R.; Casado, J.; López Navarrete, J.T.; Blanco, R.; Segura, J.L. A β-Naphthaleneimide-Modified Terthiophene Exhibiting Charge Transfer and Polarization through the Short Molecular Axis. Joint Spectroscopic and Theoretical Study. J. Phys. Chem. A 2008, 112, 29, 6732–6740. [Google Scholar] [CrossRef]
- Benami, A.; Santana, G.; Ortiz, A.; Ponce, A.; Romeu, D.; Aguilar-Hernández, J.; Contreras-Puente, G.; Alonso, J.C. Strong white and blue photoluminescence from silicon nanocrystals in SiNx grown by remote PECVD using SiCl4/NH3. Nanotechnology 2007, 18, 155704. [Google Scholar] [CrossRef]







| Sample | ν(C-N) | ν(C=C) | ν(C=N) | ν(C-H) | α/β- form | ν(C-S), ν(C-H), ν(C=C) | Nylon 11 ν(N-H) | Nylon 11 ν(C=O) |
|---|---|---|---|---|---|---|---|---|
| ZnPc (KBr) | 1286 | 1607 | 1332 | 1484, 1164, 1118, 1088 | 778, 724 | - | - | - |
| F16ZnPc (KBr) | 1280 | 1607 | 1332 | 1483, 1162, 1117, 1080 | 770, 720 | - | - | - |
| DBTTF (KBr) | - | - | - | - | - | 1508, 1151, 783 | - | - |
| ONs-1 (KBr) | 1282 | 1603 | 1332 | 1486, 1160, 1119, 1080 | 776, 718 | 1508, 1152, 778 | - | - |
| ONs-1 membrane | 1283 | - | 1336 | 1484, 1166, 1119, 1080 | - | 1156 | 3306, 3085 | 1656 |
| ONs-2 (KBr) | 1287 | 1609 | 1331 | 1486, 1164, 1115, 1085 | 773, 723 | 1508, 1152, 777 | - | - |
| ONs-2 membrane | 1287 | - | 1336 | 1484, 1165, 1118, 1080 | - | 1504, 1150, 778 | 3306, 3083 | 1656 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Vergara, M.E.; Guevara-Martínez, E.; Arreola-Castillo, A.; Mendoza-Sevilla, A. Fabrication of Hybrid Membranes Containing Nylon-11 and Organic Semiconductor Particles with Potential Applications in Molecular Electronics. Polymers 2020, 12, 9. https://doi.org/10.3390/polym12010009
Sánchez-Vergara ME, Guevara-Martínez E, Arreola-Castillo A, Mendoza-Sevilla A. Fabrication of Hybrid Membranes Containing Nylon-11 and Organic Semiconductor Particles with Potential Applications in Molecular Electronics. Polymers. 2020; 12(1):9. https://doi.org/10.3390/polym12010009
Chicago/Turabian StyleSánchez-Vergara, María Elena, Elizabeth Guevara-Martínez, Alejandra Arreola-Castillo, and Alejandra Mendoza-Sevilla. 2020. "Fabrication of Hybrid Membranes Containing Nylon-11 and Organic Semiconductor Particles with Potential Applications in Molecular Electronics" Polymers 12, no. 1: 9. https://doi.org/10.3390/polym12010009