Effects of Graphene Nanoplatelets and Cellular Structure on the Thermal Conductivity of Polysulfone Nanocomposite Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Foaming Methods
2.3. Testing Procedure
3. Results
3.1. Cellular Structure of the Foams
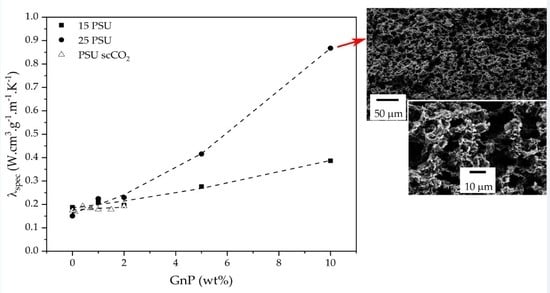
3.2. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McKeen, L.W. 12-High Temperature/High Performance Polymers. In Plastics Design Library; William Andrew Publishing: Boston, MA, USA, 2012; pp. 315–337. ISBN 978-1-4557-2551-9. [Google Scholar]
- Gohil, S.V.; Suhail, S.; Rose, J.; Vella, T.; Nair, L.S. Polymers and composites for orthopedic applications. In Materials for Bone Disorders; Elsevier: Amsterdam, The Netherlands, 2017; pp. 349–403. [Google Scholar]
- Kyriacos, D. High-Temperature Engineering Thermoplastics. In Brydson’s Plastics Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 545–615. [Google Scholar]
- Krause, B.; Diekmann, K.; van der Vegt, N.F.A.; Wessling, M. Open Nanoporous Morphologies from Polymeric Blends by Carbon Dioxide Foaming. Macromolecules 2002, 35, 1738–1745. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Y.; Bai, S. Polysulfone foam with high expansion ratio prepared by supercritical carbon dioxide assisted molding foaming method. RSC Adv. 2018, 8, 2880–2886. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Nicolae, A.; Kumar, V. Solid-state microcellular and nanocellular polysulfone foams. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 975–985. [Google Scholar] [CrossRef]
- Sun, H.; Mark, J.E. Preparation, characterization, and mechanical properties of some microcellular polysulfone foams. J. Appl. Polym. Sci. 2002, 86, 1692–1701. [Google Scholar] [CrossRef]
- Krause, B.; Boerrigter, M.E.; van der Vegt, N.F.A.; Strathmann, H.; Wessling, M. Novel open-cellular polysulfone morphologies produced with trace concentrations of solvents as pore opener. J. Memb. Sci. 2001, 187, 181–192. [Google Scholar] [CrossRef]
- Huang, Q.; Klötzer, R.; Seibig, B.; Paul, D. Extrusion of microcellular polysulfone using chemical blowing agents. J. Appl. Polym. Sci. 1998, 69, 1753–1760. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Development of novel SiO2–GO nanohybrid/polysulfone membrane with enhanced performance. J. Memb. Sci. 2014, 451, 94–102. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Memb. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
- Anadão, P.; Sato, L.F.; Wiebeck, H.; Valenzuela-Díaz, F.R. Montmorillonite as a component of polysulfone nanocomposite membranes. Appl. Clay Sci. 2010, 48, 127–132. [Google Scholar] [CrossRef]
- Devrim, Y.; Erkan, S.; Baç, N.; Eroğlu, I. Preparation and characterization of sulfonated polysulfone/titanium dioxide composite membranes for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2009, 34, 3467–3475. [Google Scholar] [CrossRef]
- Lawal, A.T. Graphene-based nano composites and their applications: A review. Biosen. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Realinho, V.; Velasco, J.I. Foaming behaviour, structure, and properties of polypropylene nanocomposites foams. J. Nanomater. 2010, 2010, 4. [Google Scholar] [CrossRef]
- King, J.A.; Tucker, K.W.; Vogt, B.D.; Weber, E.H.; Quan, C. Electrically and thermally conductive nylon 6, 6. Polym. Compos. 1999, 20, 643–654. [Google Scholar] [CrossRef]
- Verdejo, R.; Barroso-Bujans, F.; Rodriguez-Perez, M.A.; De Saja, J.A.; Lopez-Manchado, M.A. Functionalized graphene sheet filled silicone foam nanocomposites. J. Mater. Chem. 2008, 18, 2221–2226. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef] [Green Version]
- Fraleoni-Morgera, A.; Chhikara, M. Polymer-based nano-composites for thermal insulation. Adv. Eng. Mater. 2019, 21, 1801162. [Google Scholar] [CrossRef]
- Gedler, G.; Antunes, M.; Borca-Tasciuc, T.; Velasco, J.I.; Ozisik, R. Effects of graphene concentration, relative density and cellular morphology on the thermal conductivity of polycarbonate–graphene nanocomposite foams. Eur. Polym. J. 2016, 75, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, H.; Drzal, L.; Rook, B.; Rich, M. Thermal conductivity of exfoliated graphite nanocomposites. J. Therm. Anal. Calorim. 2006, 85, 235–238. [Google Scholar] [CrossRef]
- Gonnet, P.; Liang, Z.; Choi, E.S.; Kadambala, R.S.; Zhang, C.; Brooks, J.S.; Wang, B.; Kramer, L. Thermal conductivity of magnetically aligned carbon nanotube buckypapers and nanocomposites. Curr. Appl. Phys. 2006, 6, 119–122. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Lv, Z.; Li, N.; Liang, C.; Zhang, Q. Functionalized graphite nanoplatelets/epoxy resin nanocomposites with high thermal conductivity. Int. J. Heat Mass Transf. 2016, 92, 15–22. [Google Scholar] [CrossRef]
- Shtein, M.; Nadiv, R.; Buzaglo, M.; Kahil, K.; Regev, O. Thermally conductive graphene-polymer composites: Size, percolation, and synergy effects. Chem. Mater. 2015, 27, 2100–2106. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, E.; Li, X.; Zhang, Y.; Qu, J.; Yu, Z.-Z. Cellulose/graphene aerogel supported phase change composites with high thermal conductivity and good shape stability for thermal energy storage. Carbon 2016, 98, 50–57. [Google Scholar] [CrossRef]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Mechanical properties and thermal conductivity of graphene nanoplatelet/epoxy composites. J. Mater. Sci. 2015, 50, 1082–1093. [Google Scholar] [CrossRef]
- Hu, L.; Desai, T.; Keblinski, P. Thermal transport in graphene-based nanocomposite. J. Appl. Phys. 2011, 110, 33517. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I.I. Graphene nanoplatelets-reinforced polyetherimide foams prepared by water vapor-induced phase separation. eXPRESS Polym. Lett. 2015, 9, 412–423. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I.I. Enhancing the electrical conductivity of polyetherimide-based foams by simultaneously increasing the porosity and graphene nanoplatelets dispersion. Polym. Compos. 2018, 40, E1416–E1425. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Polyetherimide foams filled with low content of graphene nanoplatelets prepared by scCO2 dissolution. Polymers 2019, 11, 328. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Effects of Carbon Nanotubes/Graphene Nanoplatelets Hybrid Systems on the Structure and Properties of Polyetherimide-Based Foams. Polymers 2018, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.Z.; Chung, T.-S.; Lai, J.-Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Sims, G.L.A.; Khunniteekool, C. Cell-size measurement of polymeric foams. Cell. Polym. 1994, 13, 137–146. [Google Scholar]
- Glicksman, L.R. Heat transfer in foams. In Low Density Cellular Plastics. Physical Basis of Behaviour; Chapman & Hall: London, UK, 1994; pp. 104–152. [Google Scholar]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999; ISBN 131602542X. [Google Scholar]
- Holman, J.P. Heat Transfer; McGraw-Hill: New York, NY, USA, 2010; ISBN 0071267697. [Google Scholar]
- Weast, R.C.; Astle, M.J.; Beyer, W.H. Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1988; Volume 69. [Google Scholar]














| Foam Series | Foam Code | Density (g/cm3) | Relative Density | Vg | VPSU | VGnP | Φ (μm) | N0 (cells/cm3) | Nf (cells/cm3) |
|---|---|---|---|---|---|---|---|---|---|
| Series 1 | 15 PSU | 0.299 | 0.24 | 0.759 | 0.241 | 0.000 | 29.7 | 1.9 × 108 | 5.5 × 107 |
| 15 PSU 1 GnP | 0.351 | 0.28 | 0.719 | 0.280 | 0.002 | 55.5 | 3.3 × 107 | 8.1 × 106 | |
| 15 PSU 2 GnP | 0.370 | 0.30 | 0.705 | 0.292 | 0.003 | 56.7 | 3.2 × 107 | 7.5 × 106 | |
| 15 PSU 5 GnP | 0.402 | 0.32 | 0.683 | 0.308 | 0.009 | 50.1 | 5.1 × 107 | 1.0 × 107 | |
| 15 PSU 10 GnP | 0.500 | 0.39 | 0.615 | 0.362 | 0.023 | Open cell | - | - | |
| Series 2 | 25 PSU | 0.435 | 0.35 | 0.649 | 0.351 | 0.000 | 19.9 | 5.2 × 108 | 1.5 × 108 |
| 25 PSU 1 GnP | 0.402 | 0.32 | 0.677 | 0.321 | 0.002 | 21.2 | 3.7 × 108 | 1.3 ×108 | |
| 25 PSU 2 GnP | 0.507 | 0.41 | 0.595 | 0.401 | 0.005 | 33.9 | 1.0 × 108 | 2.9 × 107 | |
| 25 PSU 5 GnP | 0.532 | 0.42 | 0.580 | 0.408 | 0.012 | Open cell | - | - | |
| 25 PSU 10 GnP | 0.588 | 0.45 | 0.547 | 0.426 | 0.027 | Open cell | - | - | |
| Series 3 | PSU | 0.443 | 0.36 | 0.643 | 0.357 | 0.000 | 19.1 | 2.1 × 108 | 1.8 × 108 |
| PSU 0.1 GnP | 0.428 | 0.35 | 0.655 | 0.345 | 0.000 | 17.3 | 2.5 × 108 | 2.4 × 108 | |
| PSU 0.4 GnP | 0.457 | 0.37 | 0.632 | 0.367 | 0.001 | 13.9 | 3.4 × 108 | 4.5 × 108 | |
| PSU 0.7 GnP | 0.472 | 0.38 | 0.620 | 0.378 | 0.002 | 15.1 | 2.7 × 108 | 3.5 × 108 | |
| PSU 1 GnP | 0.510 | 0.41 | 0.590 | 0.407 | 0.002 | 13.1 | 4.1 × 108 | 5.1 × 108 | |
| PSU 1.5 GnP | 0.575 | 0.46 | 0.539 | 0.457 | 0.004 | 13.8 | 3.7 × 108 | 4.0 × 108 | |
| PSU 2 GnP | 0.561 | 0.45 | 0.552 | 0.443 | 0.005 | 14.9 | 2.1 × 108 | 3.1 × 108 |
| Foam Series | Foam Code | Relative Density | λexp (W·m−1·K−1) |
|---|---|---|---|
| Series 1 | 15 PSU | 0.24 | 0.056 |
| 15 PSU 1 GnP | 0.28 | 0.073 | |
| 15 PSU 2 GnP | 0.30 | 0.073 | |
| 15 PSU 5 GnP | 0.32 | 0.111 | |
| 15 PSU 10 GnP | 0.39 | 0.193 | |
| Series 2 | 25 PSU | 0.35 | 0.066 |
| 25 PSU 1 GnP | 0.32 | 0.090 | |
| 25 PSU 2 GnP | 0.41 | 0.116 | |
| 25 PSU 5 GnP | 0.42 | 0.221 | |
| 25 PSU 10 GnP | 0.45 | 0.510 | |
| Series 3 | PSU | 0.36 | 0.077 |
| PSU 0.1 GnP | 0.35 | 0.072 | |
| PSU 0.4 GnP | 0.37 | 0.088 | |
| PSU 0.7 GnP | 0.38 | 0.087 | |
| PSU 1 GnP | 0.41 | 0.091 | |
| PSU 1.5 GnP | 0.46 | 0.102 | |
| PSU 2 GnP | 0.45 | 0.108 |
| Foam Series | GnP (wt%) | ξλc (W·m−1·K−1) | Relative Density | n |
|---|---|---|---|---|
| Series 1 | 0 | 0.150 | 0.24 | 0.692 |
| 1 | 0.192 | 0.28 | 0.764 | |
| 2 | 0.184 | 0.30 | 0.760 | |
| 5 | 0.294 | 0.32 | 0.847 | |
| 10 | 0.459 | 0.39 | 0.909 | |
| Series 2 | 0 | 0.138 | 0.35 | 0.712 |
| 1 | 0.225 | 0.32 | 0.806 | |
| 2 | 0.248 | 0.41 | 0.840 | |
| 5 | 0.490 | 0.42 | 0.918 | |
| 10 | 1.093 | 0.45 | 0.964 | |
| Series 3 | 0 | 0.168 | 0.36 | 0.760 |
| 0.1 | 0.159 | 0.35 | 0.744 | |
| 0.4 | 0.194 | 0.37 | 0.791 | |
| 0.7 | 0.185 | 0.38 | 0.785 | |
| 1.0 | 0.185 | 0.41 | 0.791 | |
| 1.5 | 0.192 | 0.46 | 0.808 | |
| 2.0 | 0.209 | 0.45 | 0.821 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, H.; Antunes, M.; Velasco, J.I. Effects of Graphene Nanoplatelets and Cellular Structure on the Thermal Conductivity of Polysulfone Nanocomposite Foams. Polymers 2020, 12, 25. https://doi.org/10.3390/polym12010025
Abbasi H, Antunes M, Velasco JI. Effects of Graphene Nanoplatelets and Cellular Structure on the Thermal Conductivity of Polysulfone Nanocomposite Foams. Polymers. 2020; 12(1):25. https://doi.org/10.3390/polym12010025
Chicago/Turabian StyleAbbasi, Hooman, Marcelo Antunes, and José Ignacio Velasco. 2020. "Effects of Graphene Nanoplatelets and Cellular Structure on the Thermal Conductivity of Polysulfone Nanocomposite Foams" Polymers 12, no. 1: 25. https://doi.org/10.3390/polym12010025