Polystyrene Nanocomposites Reinforced with Novel Dumbbell-Shaped Phenyl-POSSs: Synthesis and Thermal Characterization
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. 1H NMR Spectroscopy
2.3. Scanning Electron Microscopy (SEM)
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Thermogravimetric Analysis (TGA)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Dalla Rosa, M. Gas permeability and thermal behavior of polypropylene films used for packaging minimally processed fresh-Cut potatoes: A case study. J. Food Sci. 2012, 77, 264. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Review: Raw Natural Fiber–Based Polymer Composites. Int. J. Polym. Anal. Charact. 2013, 18, 495. [Google Scholar] [CrossRef]
- Peplow, M. The plastics revolution: How chemists are pushing polymers to new limits. Nature 2016, 536, 266. [Google Scholar] [CrossRef] [PubMed]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Adamus-Włodarczyk, A.; Kairytė, A.; Vaitkus, S. Composites of Rigid Polyurethane Foams Reinforced with POSS. Polymers 2019, 11, 336. [Google Scholar] [CrossRef]
- Cavallaro, G.; Grillo, I.; Gradzielski, M.; Lazzara, G. Structure of Hybrid Materials Based on Halloysite Nanotubes Filled with Anionic Surfactants. J. Phys. Chem. C 2016, 120, 13492. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Konnova, S.; Fakhrullin, R.; Lvov, Y. Composite films of natural clay nanotubes with cellulose and chitosan. Green Mater. 2014, 2, 232–242. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Veronesi, P.; Lamanna, G. Influence of PCL on mechanical properties and bioactivity of ZrO2-Based hybrid coatings synthesized by sol-Gel dip coating technique. Mater. Sci. Eng. C 2014, 39, 344. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Zheng, Y.X.; Walter, R.; Friedrich, K. Structure–Property relationships of irradiation grafted nano-Inorganic particle filled polypropylene composites. Polymer 2001, 42, 167. [Google Scholar] [CrossRef]
- Shvartzman-Cohen, R.; Nativ-Roth, E.; Baskaran, E.; Szleifer, I.; Yerushalmi-Rozen, R. Selective dispersion of single-Walled carbon nanotubes in the presence of polymers: The role of molecular and colloidal length scales. J. Am. Chem. Soc. 2004, 126, 14850. [Google Scholar] [CrossRef]
- Monticelli, O.; Fina, A.; Ullah, A.; Waghmare, P. Preparation, Characterization, and Properties of Novel PSMA−POSS Systems by Reactive Blending. Macromolecules 2009, 42, 6614. [Google Scholar] [CrossRef]
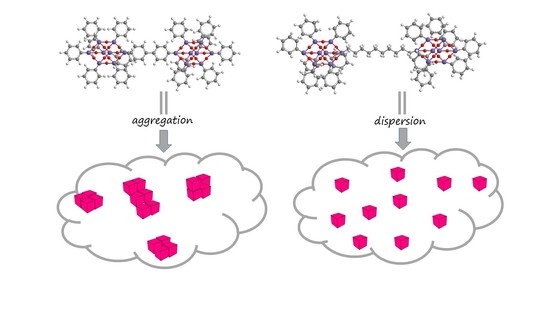
- Blanco, I.; Bottino, F.A.; Cicala, G.; Cozzo, G.; Latteri, A.; Recca, A. Synthesis and thermal characterization of new dumbbell shaped POSS/PS nanocomposites: Influence of the symmetrical structure of the nanoparticles on the dispersion/aggregation in the polymer matrix. Polym. Compos. 2015, 36, 1394. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A.; Bottino, P. Synthesis, characterization and thermal stability of new dumbbell-shaped isobutyl-Substituted POSSs linked by aromatic bridges. J. Therm. Anal. Calorim. 2014, 117, 243. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, X.; Wu, Q.; Han, J.; Jiang, J. Assembly of Polyacrylamide-Sodium Alginate-Based Organic-Inorganic Hydrogel with Mechanical and Adsorption Properties. Polymers 2019, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, X.H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Cha, C.G.; Yu, Y.G.; Seo, H.B.; Kim, M.J.; Grubbs, R.H.; Lee, J.S. Experimental Formulation of Photonic Crystal Properties for Hierarchically Self-Assembled POSS–Bottlebrush Block Copolymers. Macromolecules 2018, 51, 3458–3466. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Y.; Li, Z.; Nan, N.; Zhu, Y.; Li, Y. Froth flotation giant surfactants. Polymer 2019, 162, 58–62. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. Synthesis and characterization of differently substituted phenyl hepta isobutyl-Polyhedral oligomeric silsesquioxane/polystyrene nanocomposites. Polym. Compos. 2014, 35, 151. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Abate, L. Influence of n-Alkyl substituents on the thermal behaviour of Polyhedral Oligomeric Silsesquioxanes (POSSs) with different cage’s periphery. Thermochim. Acta 2016, 623, 50. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A. Mono substituted octaphenyl POSSs: The effects of substituents on thermal properties and solubility. Thermochim. Acta 2017, 655, 117. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Lichtenhan, J.D. Polyhedral Oligomeric Silsesquioxanes: Building Blocks for Silsesquioxane-Based Polymers and Hybrid Materials. Comments Inorg. Chem. 1995, 17, 115–130. [Google Scholar] [CrossRef]
- Haddad, T.S.; Lichtenhan, J.D. Hybrid organic-Inorganic thermoplastics: Styryl-Based polyhedral oligomeric silsesquioxane polymers. Macromolecules 1996, 29, 7302–7304. [Google Scholar] [CrossRef]
- Kalia, S.; Pielichowski, K. Polymer/POSS Nanocomposites and Hybrid Materials. In Preparation, Properties, Applications; Springer Nature: Basel, Switzerland, 2019. [Google Scholar]
- Wei, W.; Zhang, Y.; Liu, M.; Zhang, Y.; Yin, Y.; Gutowski, W.S.; Deng, P.; Zheng, C. Improving the Damping Properties of Nanocomposites by Monodispersed Hybrid POSS Nanoparticles: Preparation and Mechanisms. Polymers 2019, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Abate, L.; Bottino, F.A.; Bottino, P.; Chiacchio, M.A. Thermal degradation of differently substituted Cyclopentyl Polyhedral Oligomeric Silsesquioxane (CP-POSS) nanoparticles. J. Therm. Anal. Calorim. 2012, 107, 1083–1091. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A.; Bottino, P. Hepta isobutyl polyhedral oligomeric silsesquioxanes (hib-POSS): A thermal degradation study. J. Therm. Anal. Calorim. 2012, 108, 807–815. [Google Scholar] [CrossRef]
- Puglisi, C.; Sturiale, L.; Montaudo, G. Thermal Decomposition Processes in Aromatic Polycarbonates Investigated by Mass Spectrometry. Macromolecules 1999, 327, 2194–2203. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E. The effect of phenyl content on the degradation of poly(dimethyl diphenyl) siloxane copolymers. Polym. Degrad. Stabil. 2001, 74, 363–370. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Carniato, F.; Frache, A.; Boccaleri, E.; Camino, G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim. Acta 2006, 440, 36. [Google Scholar] [CrossRef]
- Shea, K.J.; Loy, D.A. Bridged polysilsesquioxanes. Molecular-Engineered hybrid organic–Inorganic materials. Chem. Mater. 2001, 13, 3306–3319. [Google Scholar] [CrossRef]
- Araki, H.; Naka, K. Syntheses and properties of dumbbell-Shaped POSS derivatives linked by luminescent π-conjugated units. J. Polym. Sci. Part A 2012, 50, 4170–4181. [Google Scholar] [CrossRef]
- Araki, H.; Naka, K. Syntheses and properties of star- and dumbbell-shaped POSS derivatives containing isobutyl groups. Polym. J. 2012, 44, 340–346. [Google Scholar] [CrossRef]
- Hunks, W.J.; Ozin, G.A. Periodic mesoporous phenylenesilicas with ether or sulfide hinge groups—A new class of PMOs with ligand channels. Chem. Commun. 2004, 21, 2426–2427. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Abate, L.; Bottino, F.A. Synthesis and thermal behaviour of phenyl-substituted POSSs linked by aliphatic and aromatic bridges. J. Therm. Anal. Calorim. 2018, 131, 843. [Google Scholar] [CrossRef]
- Badea, E.; Blanco, I.; Della Gatta, G. Fusion and solid-To-Solid transitions of a homologous series of alkane α, ω dinitriles. J. Chem. Thermodyn. 2007, 39, 1392–1398. [Google Scholar] [CrossRef]
- Della Gatta, G.; Richardson, M.J.; Sarge, S.M.; Stølen, S. Standards, calibration, and guidelines in microcalorimetry. Part 2. Calibration standards for differential scanning calorimetry (IUPAC Technical Report). Pure Appl. Chem. 2006, 78, 1455–1476. [Google Scholar] [CrossRef]
- Abate, L.; Blanco, I.; Cicala, G.; Recca, G.; Scamporrino, A. The influence of chain-Ends on the thermal and rheological properties of some 40/60 PES/PEES copolymers. Polym. Eng. Sci. 2009, 49, 1477. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee Recommendations for Collecting Experimental Thermal Analysis Data for Kinetic Computations. Thermochim. Acta 2014, 590, 1. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A.; Chiacchio, M.A. Synthesis and Thermal Behaviour of Novel Aliphatic/Aromatic Hepta-Cyclopentyl Bridged Polyhedral Oligomeric Silsesquioxanes (POSSs)/Polystyrene (PS) Nanocomposites. J. Inorg. Organomet. Polym. Mater. 2015, 25, 1456. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Pielichowski, K. Segmental dynamics in hybrid polymer/POSS nanomaterials. Prog. Polym. Sci. 2016, 52, 36. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A. Thermal characterization of a series of novel hepta cyclopentyl bridged POSS/PS nanocomposites. J. Therm. Anal. Calorim. 2016, 125, 637. [Google Scholar] [CrossRef]












| C86H74Si16O24 (1) | C90H82 Si16O24 (2) |
 |  |
| C94H90 Si16O2 (3) | |
 | |
| C96H78 Si16O25 (4) |
 |
| C96H78 Si16O24 (5) |
 |
| Compounds | POSS% | Tg% °C | Static Air | Nitrogen Flow | ||
|---|---|---|---|---|---|---|
| T5% °C | Residue % | T5% °C | Residue % | |||
| PS | / | 101.2 | 309.0 | 0 | 341.3 | 0 |
| 1 | 5.96 | 102.6 | 375.7 | 11.6 | 360.0 | 7.1 |
| 2 | 5.82 | 103.5 | 370.3 | 7.8 | 359.3 | 7.9 |
| 3 | 5.12 | 101.7 | 364.7 | 9.4 | 355.3 | 3.1 |
| 4 | 6.01 | 103.5 | 363.2 | 13.3 | 374.8 | 10.4 |
| 5 | 5.60 | 102.5 | 360.3 | 17.3 | 375.7 | 19.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abate, L.; Bottino, F.A.; Cicala, G.; Chiacchio, M.A.; Ognibene, G.; Blanco, I. Polystyrene Nanocomposites Reinforced with Novel Dumbbell-Shaped Phenyl-POSSs: Synthesis and Thermal Characterization. Polymers 2019, 11, 1475. https://doi.org/10.3390/polym11091475
Abate L, Bottino FA, Cicala G, Chiacchio MA, Ognibene G, Blanco I. Polystyrene Nanocomposites Reinforced with Novel Dumbbell-Shaped Phenyl-POSSs: Synthesis and Thermal Characterization. Polymers. 2019; 11(9):1475. https://doi.org/10.3390/polym11091475
Chicago/Turabian StyleAbate, Lorenzo, Francesco Agatino Bottino, Gianluca Cicala, Maria Assunta Chiacchio, Giulia Ognibene, and Ignazio Blanco. 2019. "Polystyrene Nanocomposites Reinforced with Novel Dumbbell-Shaped Phenyl-POSSs: Synthesis and Thermal Characterization" Polymers 11, no. 9: 1475. https://doi.org/10.3390/polym11091475