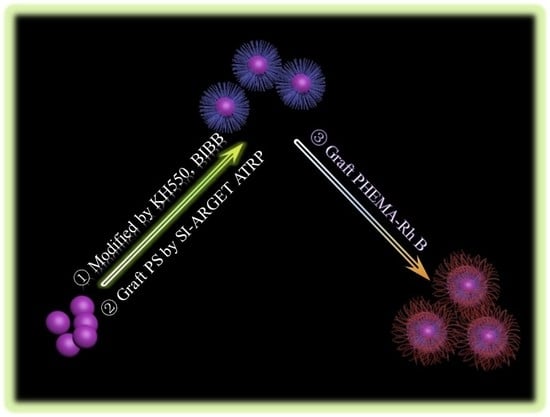
Surface-Induced ARGET ATRP for Silicon Nanoparticles with Fluorescent Polymer Brushes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumental Characterization
2.3. Preparation of the Hydrophilic Monomer with Rhodamine B (HEMA-RhB)
2.4. Anchoring of the ARGET ATRP Initiator (Bromo-Initiator) on SNPs Surfaces
2.5. Synthesis of Silica-Grafting-Polystyrene (SNPs-g-PS)
2.6. Preparation of the Fluorescence SNPs-g-PS-b-PHEMA-co-PHEMA-RHB Via SI-ARGET ATRP
3. Results and Discussion
3.1. Structure Analysis
3.2. Morphology Analysis
3.3. TGA Analysis
3.4. Fluorescence and Dispersibility Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sikora, J.W.; Gajdoš, I.; Puszka, A. Polyethylene-Matrix Composites with Halloysite Nanotubes with Enhanced Physical/Termal Properties. Polymers 2019, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, W.; Ma, Z.; Deng, Y.; Chen, Y.; Chen, Y.; Hu, W. Enhanced optomechanical properties of mechanochemiluminescent poly (methyl acrylate) composites with granulated fluorescent conjugated microporous polymer fillers. Chem. Sci. 2019, 10, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Du, X.; Yu, J.; Qin, S.; He, M.; Zhang, K.; Yang, J. Synthesis of Negatively Charged Polyol-Functional PSF Membranes with Good Hydrophilic and Efficient Boron Removal Properties. Polymers 2019, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, J.; Zhao, J.; Wang, Z.; Wang, X. In-situ active formation of carbides coated with NPTiO2 nanoparticles for efficient adsorption-photocatalytic inactivation of harmful algae in eutrophic water. Chemosphere 2019, 228, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Shchipunov, Y.A.; Karpenko, T.Y. Hybrid polysaccharide−silica nanocomposites prepared by the sol−gel technique. Langmuir 2004, 20, 3882–3887. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.Y.; Wuu, D.S.; Yeh, W.C.; Liu, J.C. Tri-layer antireflection coatings (SiO2/SiO2–TiO2/TiO2) for silicon solar cells using a sol–gel technique. Sol. Energy Mater. Sol. Cells 2006, 90, 2710–2719. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Z.; Shi, L.; Ma, Y.; Yuan, S.; Sun, L.; Zhao, Y.; Zhang, M.; Zhu, J. Layer-by-layer deposition of organic–inorganic hybrid multilayer on microporous polyethylene separator to enhance the electrochemical performance of lithium-ion battery. ACS Appl. Mater. Interfaces 2015, 7, 20678–20686. [Google Scholar] [CrossRef]
- Wu, W.; Niu, H.; Yang, D.; Wang, S.; Jiang, N.; Wang, J.; Hu, C. Polyaniline/Carbon Nanotubes composite modified anode via graft polymerization and self-assembling for microbial fuel cells. Polymers 2018, 10, 759. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Jia, H.; Yang, G.; Di, Y.; Xu, C.; Ma, L.; Zhang, H.; Zhou, Y.; Zang, Y.; et al. A novel silane coupling agent with peroxy groups used as an initiator in the graft polymerization of AN or MMA on nano-TiO2. Chem. Pap. 2018, 72, 2871–2877. [Google Scholar] [CrossRef]
- Salmi-Mani, H.; Terreros, G.; Barroca-Aubry, N.; Aymes-Chodur, C.; Regeard, C.; Roger, P. Poly (ethylene terephthalate) films modified by UV-induced surface graft polymerization of vanillin derived monomer for antibacterial activity. Eur. Polym. J. 2018, 103, 51–58. [Google Scholar] [CrossRef]
- Schulz, A.S.; Gojzewski, H.; Huskens, J.; Vos, W.L.; Julius, V.G. Controlled sub-10-nanometer poly (N-isopropyl-acrylamide) layers grafted from silicon by atom transfer radical polymerization. Polym. Adv. Technol. 2018, 29, 806–813. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, M.; Wang, T.; Xiong, L.; Hang, T.; Ling, H.; Li, M. Design of thermally stable insulation film by radical grafting poly (methylacrylic acid) on silicon surface. Appl. Surf. Sci. 2019, 464, 627–635. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, H.; Jia, Y.; Liu, J.; Zhang, H.; Wang, R.; Zhang, B.; Zhang, H.; Zhang, Q. Design and preparation of biomimetic polydimethylsiloxane (PDMS) films with superhydrophobic, self-healing and drag reduction properties via replication of shark skin and SI-ATRP. Chem. Eng. J. 2019, 356, 318–328. [Google Scholar] [CrossRef]
- Yan, C.N.; Liu, Q.; Xu, L.; Bai, L.P.; Wang, L.P.; Li, G. Photoinduced Metal-Free Surface Initiated ATRP from Hollow Spheres Surface. Polymers 2019, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wang, W.; Chen, H.; Bai, L.; Yang, H.; Wei, D.; Yang, L.; Xue, Z.; Niu, Y. Self-healing and tough GO-supported hydrogels prepared via surface-initiated ATRP and photocatalytic modification. New J. Chem. 2019, 43, 3099–3110. [Google Scholar] [CrossRef]
- He, S.; Wang, H.; Zhang, C.; Zhang, S.; Yu, Y.; Lee, Y.; Li, T. A generalizable method for the construction of MOF@ polymer functional composites through surface-initiated atom transfer radical polymerization. Chem. Sci. 2019, 10, 1816–1822. [Google Scholar] [CrossRef]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schuwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.A. Polymer brushes via surface-initiated controlled radical polymerization: Synthesis, characterization, properties, and applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, S.; Osborne, V.L.; Huck, W.T. Polymer brushes via surface-initiated polymerizations. Chem. Soc. Rev. 2004, 33, 14–22. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Synthesis of molecular brushes by “grafting onto” method: Combination of ATRP and click reactions. J. Am. Chem. Soc. 2007, 129, 6633–6639. [Google Scholar] [CrossRef]
- Simakova, A.; Averick, S.E.; Konkolewicz, D.; Matyjaszewski, K. Aqueous arget atrp. Macromolecules 2012, 45, 6371–6379. [Google Scholar] [CrossRef]
- Wang, S.; Song, J.; Li, Y.; Zhao, X.; Chen, L.; Li, G.; Wang, L.; Jia, Z.; Ge, X. Grafting antibacterial polymer brushes from titanium surface via polydopamine chemistry and activators regenerated by electron transfer ATRP. React. Funct. Polym. 2019, 140, 48–55. [Google Scholar] [CrossRef]
- Guo, M.; Wu, Y.; Xue, S.; Xia, Y.; Zhang, R.; Liu, D.; Zhang, T. Surface modification of boron nitride nanosheets with polycationic electrolytes through ARGET ATRP for enhancing mechanical properties of cellulose film. Mater. Lett. 2019, 242, 127–130. [Google Scholar] [CrossRef]
- Dong, H.; Matyjaszewski, K. ARGET ATRP of 2-(dimethylamino) ethyl methacrylate as an intrinsic reducing agent. Macromolecules 2008, 41, 6868–6870. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, H.; Zhou, L.; Zhang, F. Confined polymerization: ARGET ATRP of MMA in the nanoporesof modified SBA-15. Polymer 2017, 114, 180–188. [Google Scholar] [CrossRef]
- Ghasabkolaei, N.; Janalizadeh, A.; Jahanshahi, M.; Roshan, N.; Ghasemi, S.E. Physical and geotechnical properties of cement-treated clayey soil using silica nanoparticles: An experimental study. Eur. Phys. J. Plus 2016, 131, 134. [Google Scholar] [CrossRef]
- Wu, K.C.W.; Yamauchi, Y. Controlling physical features of mesoporous silica nanoparticles (MSNs) for emerging applications. J. Mater. Chem. 2012, 22, 1251–1256. [Google Scholar] [CrossRef]
- Dwivedi, S.; Sakamoto, S.; Kato, S.; Mitsumata, T.; Kaneko, T. Effects of biopolyimide molecular design on their silica hybrids thermo-mechanical, optical and electrical properties. RSC Adv. 2018, 8, 14009–14016. [Google Scholar] [CrossRef] [Green Version]
- Ghiyasi, S.; Sari, M.G.; Shabanian, M.; Hajibeygi, M.; Zarrintaj, P.; Rallini, M.; Torre, L.; Puglia, D.; Vahabi, H.; Jouyandeh, M.; et al. Hyperbranched poly (ethyleneimine) physically attached to silica nanoparticles to facilitate curing of epoxy nanocomposite coatings. Prog. Org. Coat. 2018, 120, 100–109. [Google Scholar] [CrossRef]
- Chen, B.K.; Chiu, T.M.; Tsay, S.Y. Synthesis and characterization of polyimide/silica hybrid nanocomposites. J. Appl. Polym. Sci. 2004, 94, 382–393. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.K.; Lee, J.K.; Jeong, Y.M.; Cheong, S.I.; Ahn, Y.C.; Kim, S.H. Production and dispersion stability of nanoparticles in nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Cai, Y.; Peng, W.; Demeshko, S.; Tian, J.; Vana, P. Silica-Coated Magnetite Nanoparticles Carrying a High-Density Polymer Brush Shell of Hydrophilic Polymer. Macromol. Rapid Commun. 2018, 39, 1800226. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Shang, L. Smart Delivery Systems Based on Poly (glycidyl methacrylate) s-Coated Organic/Inorganic Core–Shell Nanohybrids. Macromol. Rapid Commun. 2019, 1800879. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Ji, J.; Shen, J. Synthesis of near-infrared responsive gold nanorod/pnipaam core/shell nanohybrids via surface initiated atrp for smart drug delivery. Macromol. Rapid Commun. 2008, 29, 645–650. [Google Scholar] [CrossRef]
- Dai, J.; Dong, Y.; Yu, C.; Liu, Y.; Teng, X. A novel Nafion-g-PSBMA membrane prepared by grafting zwitterionic SBMA onto Nafion via SI-ATRP for vanadium redox flow battery application. J. Membr. Sci. 2018, 554, 324–330. [Google Scholar] [CrossRef]
- Guo, Q.; Han, Y.; Wang, H.; Sun, W.; Jiang, H.; Zhu, Y.; Xie, K. Thermo and electrochemical-stable composite gel polymer electrolytes derived from core-shell silica nanoparticles and ionic liquid for rechargeable lithium metal batteries. Electrochim. Acta 2018, 288, 101–107. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Ijaz, A.; Begum, R.; Naseem, K.; Usman, M.; Ajmal, M.; Saeed, U. Synthesis and characterization of inorganic–organic polymer microgels for catalytic reduction of 4-nitroaniline in aqueous medium. Polym. Compos. 2018, 39, 645–653. [Google Scholar] [CrossRef]
- Jamshidi, A.; Maleki, B.; Zonoz, F.M.; Tayebee, R. HPA-dendrimer functionalized magnetic nanoparticles (Fe3O4@ D-NH2-HPA) as a novel inorganic-organic hybrid and recyclable catalyst for the one-pot synthesis of highly substituted pyran derivatives. Mater. Chem. Phys. 2018, 209, 46–59. [Google Scholar] [CrossRef]
- Zhao, S.; Tao, Y.; Chen, Y.; Zhou, Y.; Li, R.; Xie, L.; Jin, P.; Ji, S. Room-temperature Synthesis of Inorganic-organic Hybrid Coated VO2 nanoparticles for Enhanced Durability and Flexible Temperature-responsive Near-infrared Modulator Application. ACS Appl. Mater. Interfaces 2019, 11, 10254–10261. [Google Scholar] [CrossRef]
- Kang, J.; Kim, D.; Wang, J.; Han, Y.; Zuidema, J.M.; Hariri, A.; Park, J.-H.; Jokerst, J.V.; Sailor, M.J. Enhanced performance of a molecular photoacoustic imaging agent by encapsulation in mesoporous silicon nanoparticles. Adv. Mater. 2018, 30, 1800512. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Bagheri, E.; Ansari, L.; Abnous, K.; Taghdisi, S.M.; Charbgoo, F.; Ramezani, M.; Alibolandi, M. Silica based hybrid materials for drug delivery and bioimaging. J. Control. Release 2018, 277, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Tas, S.; Kopeć, M.; van der Pol, R.; Cirelli, M.; de Vries, I.; Bölükbas, D.A.; Tempelman, K.; Benes, N.E.; Hempenius, M.A.; Vancso, G.J.; et al. Chain End-Functionalized Polymer Brushes with Switchable Fluorescence Response. Macromol. Chem. Phys. 2019, 220, 1800537. [Google Scholar] [CrossRef]
- Kopeć, M.; Tas, S.; Cirelli, M.; van der Pol, R.; de Vries, I.; Vancso, G.J.; de Beer, S. Fluorescent Patterns by Selective Grafting of a Telechelic Polymer. ACS Appl. Polym. Mater. 2019, 1, 136–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herberg, A.; Yu, X.; Kuckling, D. End Group Stability of Atom Transfer Radical Polymerization (ATRP)-Synthesized Poly(N-isopropylacrylamide): Perspectives for Diblock Copolymer Synthesis. Polymers 2019, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meng, H.; Li, Y.; Sun, D.; Zhan, Y.; Ge, X.; Chen, L. Polymer brushes grafted from graphene via bioinspired polydopamine chemistry and activators regenerated by electron transfer atom transfer radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 689–698. [Google Scholar] [CrossRef]
- Kaßel, M.; Gerke, J.; Ley, A.; Vana, P. Surface Modification of Wood Flour via ARGET ATRP and Its Application as Filler in Thermoplastics. Polymers 2018, 10, 354. [Google Scholar] [CrossRef] [PubMed]








| Elements | Si | C | N | O | Br | |
|---|---|---|---|---|---|---|
| Sample | ||||||
| SNPs-KH550 | 10.38 | 18.61 | 7.74 | 62.97 | 0.3 | |
| SNPs-Br | 8.93 | 20 | 7.18 | 55.82 | 8.07 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.-N.; Xu, L.; Liu, Q.-D.; Zhang, W.; Jia, R.; Liu, C.-Z.; Wang, S.-S.; Wang, L.-P.; Li, G. Surface-Induced ARGET ATRP for Silicon Nanoparticles with Fluorescent Polymer Brushes. Polymers 2019, 11, 1228. https://doi.org/10.3390/polym11071228
Yan C-N, Xu L, Liu Q-D, Zhang W, Jia R, Liu C-Z, Wang S-S, Wang L-P, Li G. Surface-Induced ARGET ATRP for Silicon Nanoparticles with Fluorescent Polymer Brushes. Polymers. 2019; 11(7):1228. https://doi.org/10.3390/polym11071228
Chicago/Turabian StyleYan, Chun-Na, Lin Xu, Qing-Di Liu, Wei Zhang, Rui Jia, Cheng-Zhi Liu, Shuang-Shuang Wang, Li-Ping Wang, and Guang Li. 2019. "Surface-Induced ARGET ATRP for Silicon Nanoparticles with Fluorescent Polymer Brushes" Polymers 11, no. 7: 1228. https://doi.org/10.3390/polym11071228